Monitoring Cardiotoxicity of Chemotherapy
Cardiac Imaging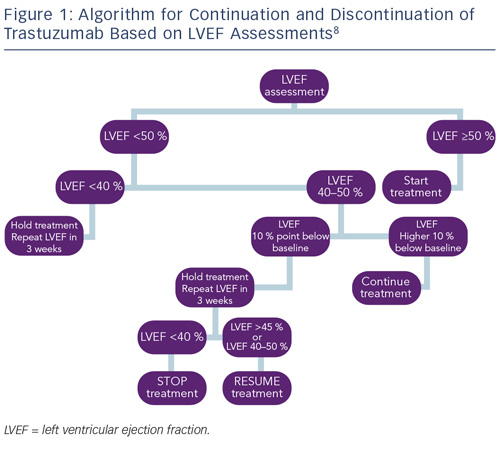
Monitoring cardiac function for early disease is recommended for all cancer survivors exposed to cardiotoxic therapies.8,83 As a result of the different timings of presentation and potential for reversibility, algorithms proposed by the European Society of Medical Oncology (ESMO) for monitoring patients exposed to anthracycline or trastuzumab have been developed to reflect these differences. Most notably, guidelines for trastuzumab incorporate advice on continuation or discontinuation of therapy and allow for the presence of potentially reversible changes (see Figure 1).
In recognition of the limitations of LVEF in detecting sub-clinical cardiotoxicity, several studies have examined the use of more sophisticated techniques, such as tissue velocity and strain imaging by echocardiography, and delayed contrast enhancement by cardiac magnetic resonance imaging (CMRI).
A recent meta-analysis of the use of myocardial strain imaging by echocardiography showed that, in late survivors of cancer, measures of global radial and circumferential strain are consistently abnormal, even in the context of normal LVEF.84 Their clinical value in predicting subsequent LVSD or HF, however, has yet to be fully explored, however, speckle tracking echocardiography (STE) and assessment of peak systolic global longitudinal strain (GLS) appears to be the most promising measure, with a 10–15 % early reduction in GLS by STE during therapy being predictive of cardiotoxicity.
Severa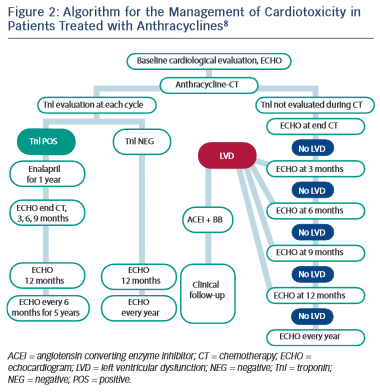 l studies have examined the use of CMRI and delayed contrast enhancement in the setting of chemotherapy-induced cardiotoxicity. While no specific changes have been identified in the anthracycline population, trastuzumab has been associated with mid-wall delayed enhancement of the lateral wall.85,86 Such findings have, however, only been reported in a small number of patients and further work is ongoing.
l studies have examined the use of CMRI and delayed contrast enhancement in the setting of chemotherapy-induced cardiotoxicity. While no specific changes have been identified in the anthracycline population, trastuzumab has been associated with mid-wall delayed enhancement of the lateral wall.85,86 Such findings have, however, only been reported in a small number of patients and further work is ongoing.
Biomarkers
Over the last two decades, data examining the use of biomarkers has demonstrated their measurement to be a more sensitive and specific tool for early identification; assessment and monitoring of chemotherapy-induced cardiac injury.69,87–89
Several studies have reported on the use of cardiac troponins during anthracycline treatment and subsequent development of LV dysfunction.88,90–92 As a result, the latest guidance from ESMO has advocated the use of troponin in routine monitoring of patients undergoing anthracycline chemotherapy (see Figure 2).8 The use of troponins to identify patients at risk of trastuzumab-induced HF or LVSD is less well defined, but has been shown to identify those at risk of developing LVSD and, among them, those less likely to recover.69,93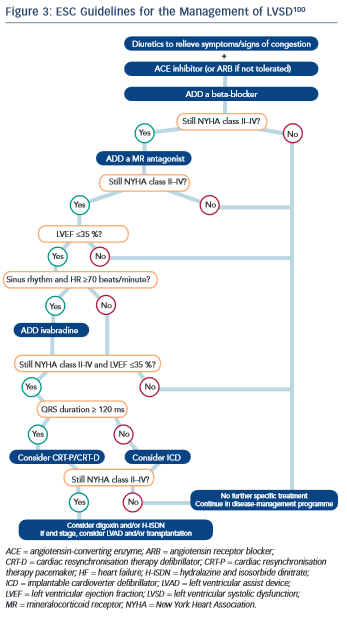
A number of studies have looked at the use of the B-type natriuretic peptides, brain natriuretic peptide/N-terminal pro-brain natriuretic peptide (BNP and NTproBNP), in predicting LVSD in both anthracycline- and trastuzumab-induced cardiotoxicity, with mixed results.94–99 Consequently, current routine use of the natriuretic peptides for monitoring cardiotoxic effects of chemotherapy remains controversial.
Treatment of Chemotherapy-induced Left Ventricular Systolic Dysfunction
To date, no guidelines regarding the treatment of LVSD have been independently validated in the oncology setting. It is widely accepted, however, that once LVSD has ensued, treatment with evidence-based medications as per national and international guidelines should be instigated (see Figure 3).8,100,101
Of the evidence that does exist in the oncology setting, most has been based on treatment of anthracycline-related LVSD. In a study of 201 adult patients, Cardinale et al. demonstrated that LV dysfunction was potentially reversible but crucially depended on the time to treatment with ACE-I and ß-blockers, supporting the need for serial monitoring.102
In contrast to LVSD secondary to anthracyclines, LVSD induced by trastuzumab is less well studied, but the initial data are promising. In a small study by Ewer, et al.,103 38 patients with a diagnosis of trastuzumab-induced LVSD all patients demonstrated recovery of LV function. Thirty-two of these were temporally associated with medical therapy including ACE-I and ß-blockers, with the remainder recovering without treatment. Of particular interest, 25 of those recovering after medical therapy were subsequently re-challenged with trastuzumab, with only three experiencing recurrence of LVSD. These results require confirmation in larger, randomised controlled trials but do indicate that monitoring may be a viable option for some patients with trastuzumab- induced cardiotoxicity and re-challenge may be ‘safe’. Moreover, several studies have demonstrated that LVSD related to trastuzumab therapy appears to be reversible, with resolution of cardiac function in approximately 80 % of cases seen in the majority of trials.73 As a result of these findings, guidelines from ESMO suggest that the introduction of prognostically indicated HF medication may not be required unless the LVEF is <40 %.8
The research was supported by the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.