History of Mechanical Circulatory Support
The first reported clinical use of a left ventricular assist device (LVAD) was by Liotta and Crawford in 1963. Via a left thoracotomy, an intracorporeal pneumatically driven pump was implanted using left atrial inflow and descending thoracic aortic outflow. Despite the successful implantation, the patient died within a short period of time after the surgery.1 A few years later, De-Bakey implanted a paracorporeal pneumatic LVAD to support the left ventricle of a woman with left ventricular failure after prior cardiac surgery. The patient recovered and could be weaned from the device successfully.2,3 The first clinical use of a total artificial heart (TAH) was reported in 1969 by Cooley. The Dacron® and Silastic® pneumatic device was placed as a bridge to transplant (BTT) in a patient who could not be weaned from cardiopulmonary bypass. The procedure was successful, heart transplantation could be performed 32 hours after the implantation, but the patient died due to pneumonia.4 Over the subsequent 20 years results after cardiac transplantation improved to modern standards, therefore mechanical circulatory support was not at the centre of investigation and clinical use was scarce, but shortage of donor organs became an increasing problem.5,6
Concerning TAH development, the Jarvik/CardioWest™ device has to be mentioned. This device would ultim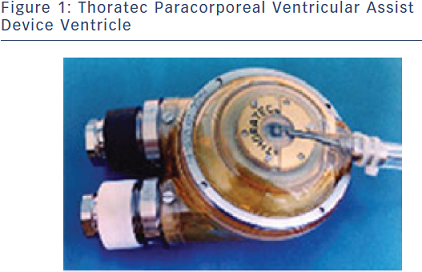 ately led to the development of today’s SynCardia TAH, which led to extreme publicity but limited clinical use.7
ately led to the development of today’s SynCardia TAH, which led to extreme publicity but limited clinical use.7
Ventricular assist devices (VADs) were initially primarily used as bridge to recovery (BTR) for patients unable to wean from cardiopulmonary bypass despite inotropic support and intra-aortic Balloon pump (IABP) or as BTT.7,8 A milestone in VAD development was the Thoratec® VAD, engineered at Penn State University. Despite the fact that this pneumatically driven pump has undergone several modifications over the years, in its basic structure it is still in clinical use today. McBride et al., published a series of BTT and BTR patients supported on the pneumatic Thoratec device. Major adverse events were: bleeding complications (31–45 %), thromboembolic events (8 %) and device-related infection (18 %). Twelve of 44 patients recovered and 39 of 67 were successfully bridged to heart transplantation (HTx).9 The Thoratec paracorporeal VAD (PVAD) is the direct descendant of this device, and is currently approved as a BTT or BTR (see Figure 1).
The more contemporary Levitronix® CentriMag® is an extracorporeal device approved for midterm support for patients in cardiogenic shock as a bridge to decision (BTD) (see Figure 2).
It is also approved for use as a right ventricular assist device (RVAD) for up to 30 days of support. In contrast to the PVAD the CentriMag is a continuous flow centrifugal pump with a magnetically levitated rotor, is preload dependent and afterload sensitive and can deliver flows of nearly 10 litres per minute. The advantages of magnetic levitation technology in blood pumps are improved durability and minimisation of blood trauma.10 The CentriMag system has provided satisfying results over the past years: the Utah Artificial Heart Program reported 83 patients (2004 to 2009), 30 RVAD, eight LVAD, 25 biventricular assist device (BiVAD) and 30 patients supported with CentriMag-driven venoarterial extracorporeal membrane oxygenation (ECMO). Survival ranged from 63 % in the LVAD group to 30 % in the veno-arterial (V-A) ECMO group. There were no device failures and bleeding related to anticoagulation was the most common complication.10
 Major concerns on the mentioned devices were reduced quality of life especially due to the fact that these devices were extracorporeal.With improvements in technology the pumps became smaller and the era of fully implantable devices began.
Major concerns on the mentioned devices were reduced quality of life especially due to the fact that these devices were extracorporeal.With improvements in technology the pumps became smaller and the era of fully implantable devices began.
Devices in this category have a driveline, which connects the intracorporeal device to either an external electrical power supply or a pneumatic driver. A broad variety of devices in this category were developed: The HeartMate® XVE, the HeartMate II (HMII), Micromed DeBakey, Jarvik 2000 FlowMaker®, HeartWare VAD (HVAD), DuraHeart and Berlin Heart INCOR. In the following years, long-term implantable LVADs have been studied and approved for BTT and destination therapy (DT) indications. Device development has progressed in a relatively orderly fashion in terms of both strategy for use and pump mechanism. Initially, pumps were conceived as a method of rescue and support to recovery. As experience grew and reliability improved implementation in a BTT scheme became common. Naturally, as data were acquired to support longer-term assistance and the devices themselves became more durable in general, DT implantation accelerated. As pulsatility was felt to be critical for organ recovery, initial LVAD designs featured pulsatile flow. Initial pulsatile devices were pneumatically driven and later electrically driven. Progress in the design and testing of newer continuous flow pumps was relatively rapid. Studies confirmed that pulsatile aortic flow was not required to resuscitate and maintain organ function in patients with end-stage heart failure.11–14 In addition, continuous flow LVADs were shown to provide significant benefits in objective quality of life and functional capacity.15,16