Future Outlook – Third Generation Device
HeartMate III, HeartWare MVAD® Driveline Free Devices
Currently the field of LVADs is undergoing an evolution towards smaller pumps with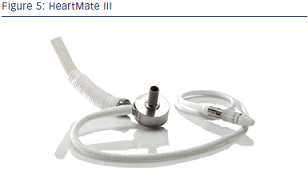 less blood trauma. Even catheter-based systems are on the horizon. The closer developments are the HeartMate III (HMIII) from Thoratec and the MVAD® from HeartWare.
less blood trauma. Even catheter-based systems are on the horizon. The closer developments are the HeartMate III (HMIII) from Thoratec and the MVAD® from HeartWare.
The HMIII (see Figure 5), a compact LVAD, has been designed and fabricated, featuring a centrifugal pump with a magnetically levitated rotor. The pump has been optimised by in vitro testing to achieve a design point of 7 litres per minute (L/min) against 135 mm Hg at high hydrodynamic efficiency (30 %) and to be capable of up to 10 L/min under such a load. Furthermore, the pump has demonstrated no mechanical failures, low haemolysis (4–10 mg/dl plasma free haemoglobin [Hb]) and low thrombogenicity during six (40, 27, 59, 42, 27 and 49 day) in vivo bovine studies.28,29 Key features include the device’s ‘bearingless’ (magnetic levitation) design, textured surfaces similar to the HeartMate XVE LVAD to reduce anticoagulation requirements and thromboembolism, a sensorless flow estimator and an induced pulse mode for achieving an increased level of pulsatility with continuous flow assistance. In vitro design verification testing is underway. Preclinical testing has been performed in calves demonstrating good in vivo performance at an average flow rate of 6 L/min (maximum: >11 L/min) and normal end-organ function and host response. Induced pulse mode demonstrated the ability to produce a physiological pulse pressure in vivo. Thirteen LVADs have achieved between 16 and 40 months of long-term in vitro reliability testing and will be continued until failure. Both percutaneous and fully implanted systems are in development, with a modular connection for upgrading without replacing the LVAD.30
 HeartWare’s MVAD pump (see Figure 6) is a continuous axial flow pump, approximately one-third the size of the HVAD pump. The MVAD pump is based on the same proprietary ‘contactless’ impeller suspension technology used in the HVAD pump, with its single moving part held in place through a combination of passive magnetic and hydrodynamic forces. In vitro and in vivo studies showed promising results. Within one in vivo study the MVAD pump was implanted in an ovine model (n=9) for 90 days. Results demonstrated the safety, reliability, haemocompatibility and biocompatibility of the MVAD pump. Nine animals were implanted for 90 ± 5 days. No complications occurred during surgical implantation. Seven of the nine animals survived until elective sacrifice. Each sheep that survived to the scheduled explant appeared physically normal, with no signs of cardiovascular or other organ compromise.31 Even a transapical implantation approach was tested.32 A new cannula configuration has been developed for transapical implantation, where the outflow cannula is positioned across the aortic valve. The two primary objectives for this feasibility study were
HeartWare’s MVAD pump (see Figure 6) is a continuous axial flow pump, approximately one-third the size of the HVAD pump. The MVAD pump is based on the same proprietary ‘contactless’ impeller suspension technology used in the HVAD pump, with its single moving part held in place through a combination of passive magnetic and hydrodynamic forces. In vitro and in vivo studies showed promising results. Within one in vivo study the MVAD pump was implanted in an ovine model (n=9) for 90 days. Results demonstrated the safety, reliability, haemocompatibility and biocompatibility of the MVAD pump. Nine animals were implanted for 90 ± 5 days. No complications occurred during surgical implantation. Seven of the nine animals survived until elective sacrifice. Each sheep that survived to the scheduled explant appeared physically normal, with no signs of cardiovascular or other organ compromise.31 Even a transapical implantation approach was tested.32 A new cannula configuration has been developed for transapical implantation, where the outflow cannula is positioned across the aortic valve. The two primary objectives for this feasibility study were 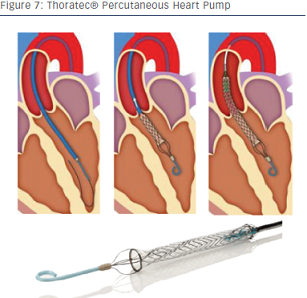 to evaluate anatomic fit and surgical approach, and efficacy of the transapical MVAD configuration. Anatomic fit and surgical approach were demonstrated using human cadavers (n=4). Efficacy was demonstrated in acute (n=2) and chronic (n=1) bovine model experiments and assessed by improvements in haemodynamics, biocompatibility, flow dynamics and histopathology. Potential advantages of the MVAD pump include flow support in the same direction as the native ventricle, elimination of cardiopulmonary bypass and minimally invasive implantation.
to evaluate anatomic fit and surgical approach, and efficacy of the transapical MVAD configuration. Anatomic fit and surgical approach were demonstrated using human cadavers (n=4). Efficacy was demonstrated in acute (n=2) and chronic (n=1) bovine model experiments and assessed by improvements in haemodynamics, biocompatibility, flow dynamics and histopathology. Potential advantages of the MVAD pump include flow support in the same direction as the native ventricle, elimination of cardiopulmonary bypass and minimally invasive implantation.
One of the major obstacles of current LVAD therapy is driveline infections. While wireless technologies have become daily routine in all our lives it is still not safe enough to run an LVAD. Eliminating of the driveline as a source of infection with VADs powered transcutaneously without wires running through an open wound will make the devices far safer. Currently all major LVAD companies and several researchers are working on this problem and experimental testing is being performed – however, it is not a clinical reality as yet.
Another approach to minimise implantation trauma is the percutaneous heart pump (PHP) by Thoratec (see Figure 7). This novel device is a fully catheter-based axial flow pump with a low profile consisting of a collapsible elastomeric impeller and nitinol cannula expandable to about 24 F. It is designed to deliver over 4 litres of flow. This device is currently under investigation. First-in-human use has already been reported.