Current Clinically Important Second Generation Devices
The Thoratec HMII (see Figure 3) is the most successful of the second generation LVAD cohort, with over 10,000 patients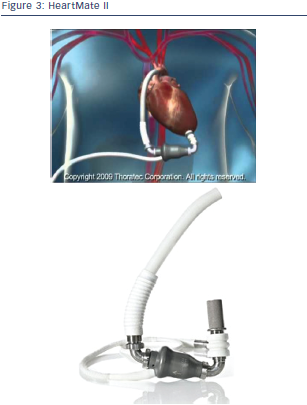 supported worldwide.17–19 It is a rotary continuous axial flow pump with an external electrical power source. Inflow cannula is inserted apically and an outflow graft anastomosed to the ascending aorta (in most of the cases) or alternatively to the subclavian artery as bailout strategy. The pump is preload dependent and afterload sensitive, runs in a fixed speed mode and is capable of up to 10 litres per minute flow at a mean aortic pressure of 100 mm mercury (Hg). The only moving part is the axial rotor, which spins on ruby ball-and-cup bearings, which are continuously washed by the flow stream. It is smaller and lighter than the HeartMate I (HMI) offering the possibility of fully intrathoracic implantation and implantations even in small adults. Recently, even minimal invasive approaches for both pump exchange and pump implantation over a subcostal incision have been described. The HMII is typically implanted into a properly sized preperitoneal pocket in the left subcostal region and utilises a driveline, which generally exits on the upper abdomen. The HMII is Food and Drug Administration (FDA) approved for both BTT and DT and has proven to be safe and effective. However, in the US the device has received approval for DT only recently, while in Europe implantation of the HMII for DT indication has already been performed for several years. The HMII BTT pivotal trial enrolled 133 patients at 26 centres in the US between March 2005 and May 2006.20 Patients were listed for transplantation as either United Network for Organ Sharing (UNOS) status IA or IB, and all had New York Heart Association (NYHA) class IV symptoms. Twenty-five percent were receiving more than one inotrope and 41 % were supported by an IABP. Seventy-five percent of patients reached the primary endpoint (number of patients who either survived to transplant, recovered and survived explant or were still alive on device) at 180 days. Fiftysix patients were transplanted, with an 80 % one-year survival. One patient recovered and had the device explanted. Twenty-five patients died before 180 days (19 %). Seventy-five percent of patients were discharged after LVAD implant; the median length of stay was 25 days. Adverse events included stroke in 11 patients (8 %), five of which occurred within the first 48 hours, device-related infection (14 %), bleeding requiring surgery (31 %) and pump thrombosis in two patients. There were no device failures, and improvements in quality of life as well as functional capacity were significant.
supported worldwide.17–19 It is a rotary continuous axial flow pump with an external electrical power source. Inflow cannula is inserted apically and an outflow graft anastomosed to the ascending aorta (in most of the cases) or alternatively to the subclavian artery as bailout strategy. The pump is preload dependent and afterload sensitive, runs in a fixed speed mode and is capable of up to 10 litres per minute flow at a mean aortic pressure of 100 mm mercury (Hg). The only moving part is the axial rotor, which spins on ruby ball-and-cup bearings, which are continuously washed by the flow stream. It is smaller and lighter than the HeartMate I (HMI) offering the possibility of fully intrathoracic implantation and implantations even in small adults. Recently, even minimal invasive approaches for both pump exchange and pump implantation over a subcostal incision have been described. The HMII is typically implanted into a properly sized preperitoneal pocket in the left subcostal region and utilises a driveline, which generally exits on the upper abdomen. The HMII is Food and Drug Administration (FDA) approved for both BTT and DT and has proven to be safe and effective. However, in the US the device has received approval for DT only recently, while in Europe implantation of the HMII for DT indication has already been performed for several years. The HMII BTT pivotal trial enrolled 133 patients at 26 centres in the US between March 2005 and May 2006.20 Patients were listed for transplantation as either United Network for Organ Sharing (UNOS) status IA or IB, and all had New York Heart Association (NYHA) class IV symptoms. Twenty-five percent were receiving more than one inotrope and 41 % were supported by an IABP. Seventy-five percent of patients reached the primary endpoint (number of patients who either survived to transplant, recovered and survived explant or were still alive on device) at 180 days. Fiftysix patients were transplanted, with an 80 % one-year survival. One patient recovered and had the device explanted. Twenty-five patients died before 180 days (19 %). Seventy-five percent of patients were discharged after LVAD implant; the median length of stay was 25 days. Adverse events included stroke in 11 patients (8 %), five of which occurred within the first 48 hours, device-related infection (14 %), bleeding requiring surgery (31 %) and pump thrombosis in two patients. There were no device failures, and improvements in quality of life as well as functional capacity were significant.
Survival of patients still on VAD support was 72 % at three years. Main complications were bleeding requiring surgical re-intervention in 26 %, driveline/pump infections in 16 %, right ventricular (RV) dysfunction in 13 % and RV failure requiring a RVAD in 6 %, 5 % had ischaemic stroke and 3 % haemorrhagic stroke. Four pumps have been removed due to thrombus. Likewise to the initial study, quality of life and functional capacity were significantly improved. The HMII was approved for BTT on the basis of the results reported above. Post-approval market analysis as required by the FDA was published in 2011. Implantation of the now commercially available HMII allowed the data to be registered by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), and the comparison group for this study was an INTERMACS cohort of 169 patients receiving another commercially available LVAD for BTT.21 The comparison group contained 135 patients with the Thoratec HeartMate XVE and 34 patients with the Thoratec IVAD, both pulsatile pumps. Ninety percent of the HMII group versus 80 % of the comparison group reached survival to transplant, survival on support or survival after device explant at six months. Overall, 12 months survival was 85 % in the HMII group versus 70 % in the comparison group. In-hospital survival in the HMII group was significantly better at 94 % compared with the comparison group at 85 %. Ninety-two percent of the HMII patients were discharged versus 75 % of the comparison group.
Adverse events in the HMII included bleeding (21.0 %), device infection (20.2 %), stroke (6.5 %), RV failure (15.0 %) and device replacement (1.2 %). The important aspects of this trial were that it confirmed the good results seen in previous studies, even in an uncontrolled setting, and it suggested that the morbidity and mortality associated with HMII implantation and support are decreasing with time. The encouraging device performance in the BTT pivotal trial resulted in FDA approval for the DT indication.22 In a separate DT trial, 38 centres in the US randomised patients 2:1 to receive either the HMII or the HeartMate XVE. Thirty-three percent of the HMII versus 41 % of the HeartMate XVE patients died within two years. In the HMII group stroke occurred in 11 % and pump replacement in 10 % compared with 36 % and 12 %, respectively in the HeartMate XVE group. The HeartMate XVE replacements were required for bearing wear, valve deterioration or infection, while broken percutaneous leads were the cause of the majority of the HMII replacements. Actuarial survival rates at one and two years for the HMII patients were 68 % and 58 % compared with 55 % and 24 % in the HeartMate XVE patients. This trial showed improved survival and complication rates in advanced heart failure patients supported with the HMII continuous flow LVAD compared with those supported with the pulsatile HeartMate XVE.
 Very low rates of pump thrombosis of the HMII has been advocated as a major advantage of the system also in comparison with other contemporary devices. However, recently a report came out showing an unexpected sudden increase in rates of pump thrombosis in HMII patients.23 It remains a matter of debate what is causing this increase (changes in anticoagulation management, variability of implantation technique, pump-related factors, patient-related factors, etc.) and it is not clear if this increase in pump thrombosis is only temporary and will return to normal rates again. Nevertheless special attention has to be paid to this phenomenon.
Very low rates of pump thrombosis of the HMII has been advocated as a major advantage of the system also in comparison with other contemporary devices. However, recently a report came out showing an unexpected sudden increase in rates of pump thrombosis in HMII patients.23 It remains a matter of debate what is causing this increase (changes in anticoagulation management, variability of implantation technique, pump-related factors, patient-related factors, etc.) and it is not clear if this increase in pump thrombosis is only temporary and will return to normal rates again. Nevertheless special attention has to be paid to this phenomenon.
The HeartWare® left ventricular assist system (LVAS) is an advanced continuous flow device, which is approved in Europe and recently for BTT indication in the US (see Figure 4).24
This centrifugal pump utilises an innovative combination of passive magnetic levitation and hydrodynamic suspension to eliminate any contact between the impeller and pump housing. There are no mechanical bearings. The HeartWare is small and designed for completely intrapericardial implantation, with inflow from the left ventricular (LV) apex and outflow via a graft to the ascending aorta (HeartWare International Inc, Framingham, MA, US).7 Like other continuous flow pumps it is preload dependent and afterload sensitive, operates at a fixed speed mode and is capable of delivering up to 10 litres per minute. Results of HeartWare trials have been encouraging. In a BTT evaluation in 50 European patients six and 24 months survival to orthotopic heart transplantation (OHT), recovery or ongoing LVAD support was 90 % and 79 %, respectively. Nine deaths were observed: three cases of sepsis, three multiple organ failures and three strokes. RV failure was seen in six cases. There was an 18 % incidence of device-related infection. (mainly driveline related) Seven devices were replaced, two for complications related to the hydrodynamic suspension mechanism and four for pump thrombus.
Anticoagulation was adjusted for an international normalised ratio (INR) of 2.5–3.5.16 Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) is a BTT trial performed at 30 American centres from 2008 to 2010 and includes 140 patients in the treatment group with end-stage heart failure listed for cardiac transplant. Results in these patients were compared with 499 patient controls from INTERMACS, who had received a LVAD as BTT during the same time period. The primary outcome was survival on the original device, survival to OHT or recovery to explant at 180 days. Success was achieved in 92.0 % of the HeartWare group versus 90.1 % of the controls. Survival at 180 days and one-year in the HeartWare group was 94.0 % and 90.6 % versus 90.2 % and 85.7 % in the controls. Adverse events included bleeding requiring surgery (15.0 %), driveline infection (10.7 %), stroke (10.0 %), RV failure (22.0 %) and pump thrombus requiring replacement (3.0 %).25 Follow-up data was presented at the 2011 meeting of the International Society for Heart and Lung Transplantation (ISHLT) and included 110 additional patients approved by the FDA on a continued access protocol (CAP). The same inclusion criteria were used but the CAP patients, based on INTERMACS classification, had more advanced heart failure. Adverse events among the total 250 patient study group were as follows: bleeding requiring surgery 9.2 %, gastrointestinal bleeding 15.6 %, ischaemic stroke 7.2 %, haemorrhagic stroke 3.2 %, driveline infections 11.6 %, RV failure 19.6 % and death by 180 days 5.0 %. Sixteen pumps developed thrombus (6.4 %), 11 were exchanged and five were treated with intracavitary tissue plasminogen activator (tPA). Seventy-eight patients were transplanted with a 93 % 180 day post-transplant survival.26 A HeartWare DT trial, ‘Evaluation of the HeartWare Ventricular Assist System for Destination Therapy of Advanced Heart Failure (ENDURANCE)’, is currently accruing patients in the US. In the US the HeartWare system is currently only approved for BTT indication, FDA approval for DT is ongoing. In Europe the HVAD is already in use for BTT as well as for DT indication.
There is some evidence that the rate of pump thrombosis in HVAD patients could be slightly higher in comparison with other contemporary devices. Therefore, some centres including our own, started to change the anticoagulation regimen. At our department we give HeartWare patients two doses of 100 mg aspirin daily in addition to the standard treatment with phenprocoumon with a target INR of 2.5.
One of the major advantages of the HeartWare device is its easy implantability and its small size. This facilitates even minimally invasive implantation of the HVAD over sternotomy sparing approaches.27