Randomised Clinical Outcome Studies Designed to Address the Clinically Important Questions
In reality the adoption of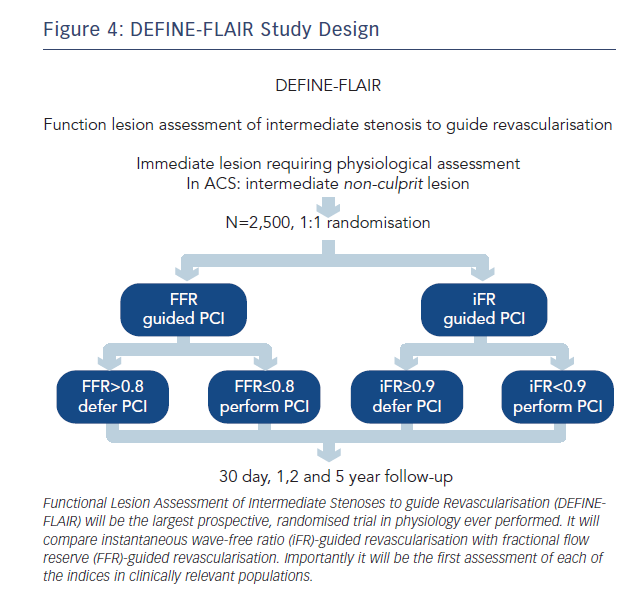
Prior to the Adenosine Vasodilator Independent Stenosis Evaluation (ADVISE) registry FFR had never been systematically studied in clinically relevant patient populations.44 The mean FFR values in the Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention (FAME) and FAME II studies were 0.71 and 0.64, respectively; significantly lower than mean FFR values in contemporary studies that have included intermediate lesions.24,44 This suggests that the FAME populations are significantly different from the patients we assess in clinical practice today.
To address this, iFR clinical outcome studies have been designed to centre on clinically relevant patient populations. Their inclusion and exclusion criteria are aimed at truly capturing the patients in whom physiology is performed in clinical practice. As a result, regardless of iFR, DEFINE-FLAIR (NCT02053038, see Figure 4) and iFR SWEDEHEART (NCT02166736) will provide the first and largest prospective real-world randomised controlled trial database for FFR in clinically relevant patient populations.
In addition there are several other unique aspects of these studies that will have direct clinical impact:
DEFINE-FLAIR and iFR SWEDEHEART will therefore provide a definitive prospective assessment of the role of both iFR and FFR in clinical practice and address some of the major uncertainties hitherto unaddressed in this field.
Regardless of the current debate upon the physiology of basal versus hyperaemia indices the data from these studies will definitively answer if hyperaemia is still required to safely assess stenosis severity and guide revascularisation. Given the simplicity and reduced uncertainty of iFR assessment they therefore have the potential to dramatically increase the adoption of physiology, finally permitting realisation of the clear health and cost benefits of such an approach.