Summary
Transcatheter Prosthetic Valve Designs, Implantation Techniques and Access Routes
As mentioned earlier, most of the currently available prostheses employ either BE or SE technologies. Although these te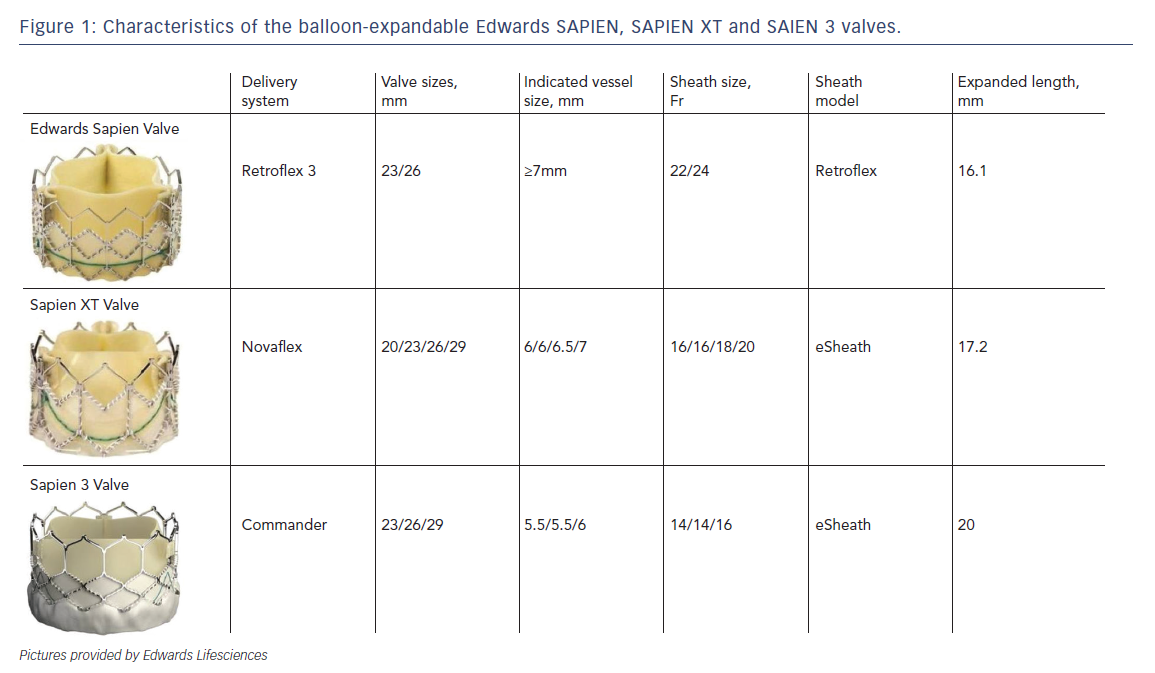 chnologies are considered broadly comparable, differences exist and device characteristics play a role in prosthesis selection. A balloon-expandable aortic stent valve, consisting of a trileaflet bovine pericardial valve tissue mounted in a stainless steel frame, was the first BE valve prototype implanted in humans.1 Subsequent improvements of the valve and the delivery systems resulted in newer generations of BE prosthesis: Edwards SAPIEN, SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA) (see Table 1, Figure 1).6 The Edwards SAPIEN valve, which was available in 23/26 mm sizes requiring 22/24 Fr delivery catheters respectively, consisted of a tubular slotted stainless steel stent frame with unidirectional trileaflet bovine pericardial tissue and a fabric skirt made of polyethylene terephthalate that improved sealing. The SAPIEN XT valve consists of a trileaflet pericardial bovine valve mounted in a cobalt chromium stent frame having fewer rows, columns and vertical struts between commissures to reduce the valve profile. This valve is available in 20/23/26/29 mm sizes, and is implanted through 16 Fr (20/23 mm valves), 18 Fr (26 mm valve) or 20 Fr (29 mm valve) expandable sheaths. The SAPIEN 3 is the latest generation of the BE valves also
chnologies are considered broadly comparable, differences exist and device characteristics play a role in prosthesis selection. A balloon-expandable aortic stent valve, consisting of a trileaflet bovine pericardial valve tissue mounted in a stainless steel frame, was the first BE valve prototype implanted in humans.1 Subsequent improvements of the valve and the delivery systems resulted in newer generations of BE prosthesis: Edwards SAPIEN, SAPIEN XT and SAPIEN 3 valves (Edwards Lifesciences, Irvine, CA) (see Table 1, Figure 1).6 The Edwards SAPIEN valve, which was available in 23/26 mm sizes requiring 22/24 Fr delivery catheters respectively, consisted of a tubular slotted stainless steel stent frame with unidirectional trileaflet bovine pericardial tissue and a fabric skirt made of polyethylene terephthalate that improved sealing. The SAPIEN XT valve consists of a trileaflet pericardial bovine valve mounted in a cobalt chromium stent frame having fewer rows, columns and vertical struts between commissures to reduce the valve profile. This valve is available in 20/23/26/29 mm sizes, and is implanted through 16 Fr (20/23 mm valves), 18 Fr (26 mm valve) or 20 Fr (29 mm valve) expandable sheaths. The SAPIEN 3 is the latest generation of the BE valves also 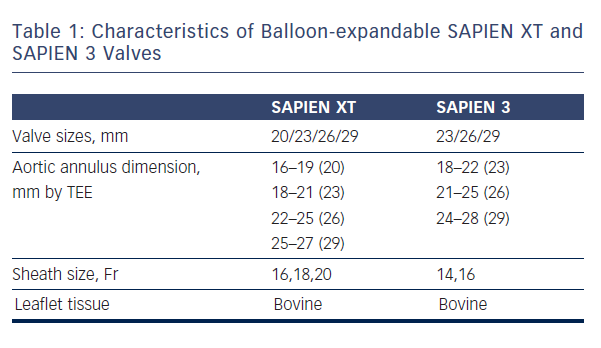 having a trileaflet pericardial bovine valve mounted in a cobalt chromium stent, but incorporating an additional outer fabric skirt to further reduce paravalvular leak.
having a trileaflet pericardial bovine valve mounted in a cobalt chromium stent, but incorporating an additional outer fabric skirt to further reduce paravalvular leak.
The CoreValve (Medtronic Inc; Minneapolis, MN), prototype of SE valves, is comprised of trileaflet porcine pericardial tissue sutured into a wire frame of nitinol, a nickel-titanium alloy that has temperature-associated shape memory features.7 The valve has three components: a high radial strength inflow segment that extends 4-6 mm below the annulus, a supra-annular segment cont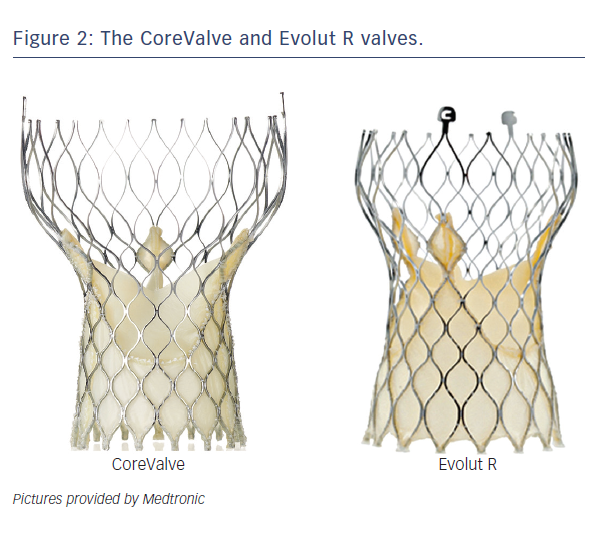 aining the leaflet, and an outflow segment that aligns the stent with the aortic root.7 The first generation SE CoreValve was available in 26/29 mm inflow diameter sizes with an 18 Fr diameter delivery system. Current generation of CoreValve is available in 23/26/29/31 mm sizes and 18 Fr delivery systems (see Table 2, Figure 2).
aining the leaflet, and an outflow segment that aligns the stent with the aortic root.7 The first generation SE CoreValve was available in 26/29 mm inflow diameter sizes with an 18 Fr diameter delivery system. Current generation of CoreValve is available in 23/26/29/31 mm sizes and 18 Fr delivery systems (see Table 2, Figure 2).
A balloon aortic valvuloplasty is usually performed prior to both SE and BE valve implantation, though this step is commonly omitted in current clinical practice. The BE valve is implanted by positioning the valve under fluoroscopy, angiography ± transoesophageal echocardiography guidance with balloon inflation for valve expansion under rapid pacing, which minimises cardiac output in order to avoid embolisation during deployment. The SE CoreValve is implanted by slow release of the valve at a marker band depth of 1-1.5 mm to obtain a final valve implantation depth of ~4 mm.7 The SE design allows partial repositionability with the newer generation dev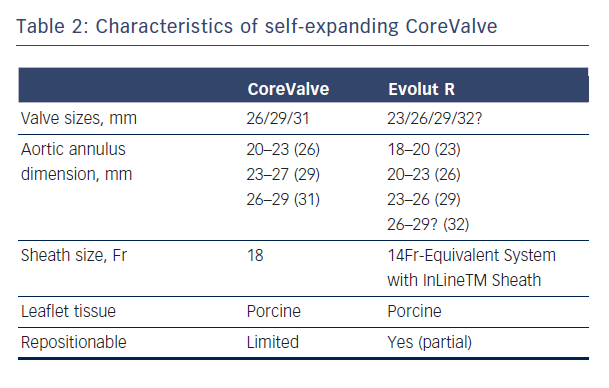 ices (such as CoreValve Evolut R, Medtronic, Minneapolis, MN, Table 1) prior to final release, as opposed to BE technology where no repositioning is possible. The newer generation SE Lotus valve allows full repositionability prior to final implantation.
ices (such as CoreValve Evolut R, Medtronic, Minneapolis, MN, Table 1) prior to final release, as opposed to BE technology where no repositioning is possible. The newer generation SE Lotus valve allows full repositionability prior to final implantation.
In the majority of patients, the retrograde transfemoral route is the preferred choice for implantation of both BE and SE prostheses.8 BE prostheses have also been delivered transapically, as well as through direct aortic access via minithoracotomy.9 The latter approaches are especially suitable in patients with severe peripheral artery disease and heavily calcified ascending aorta who are at risk of embolic complications. The SE CoreValve has also been delivered from the direct aortic in addition to the subclavian/axillary artery routes as an alternative in patients in whom the transfemoral route is contraindicated.10 The subclavian/transaxillary approach experience for BE valves is limited to a few cases.11,12



