Clinical Outcomes
Data directly comparing SE and BE valves are limited and most of the data on clinical outcomes have been generated from registries and non-comparative randomised trials (see Table 3).13–18 Furthermore, there are no long-term comparative outcome studies. To date, the multicentre Comparison of Transcatheter Heart Valves in High Risk Patients with Severe AOrtIc Stenosis: Medtronic CoreValve versus Edwards SAPIEN XT (CHOICE) trial is the only randomised clinical trial directly comparing SE and BE valves (see Table 3).20
Mortality and Stroke Rate
Data from large multicentre registries and non-comparative randomised tria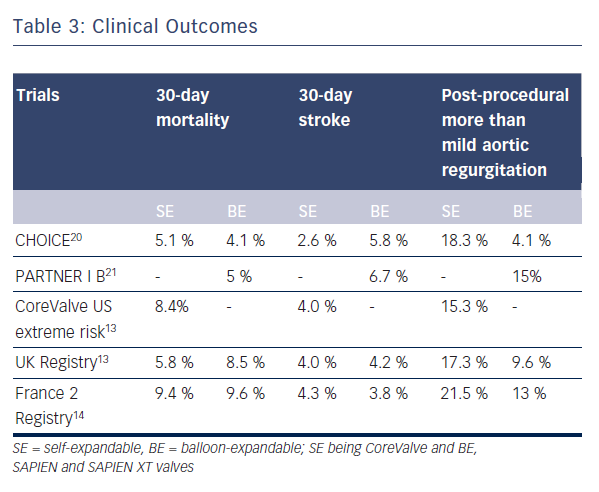
Similarly, comparative rates of embolic events were reported for SE and BE valves in several studies.27 In a large meta-analysis of neurological events after TAVI, 30-day event rates for CoreValve or SAPIEN prostheses were 3.1 % and 4.2 % respectively, the difference being non-significant.26 A statistically non-significant higher incidence of minor strokes was noted in the BE group in the CHOICE trial.20
The higher profile first generation BE SAPIEN prosthesis is likely to cause embolic events during stent positioning, whereas the lower profiled SE CoreValve may cause events mainly during stent deployment, due to shaving of calcific material from the native valve surface.28 Significantly more embolic events were observed during post-dilatation and repositioning with CoreValve as compared with BE valves.28 The unique advantage of resheathability and repositionability of modern SE valves may also translate into a higher liability to embolic events, though this remains hypothetical.
Profiles of both these devices have been improved to reduce stroke risk in newer generations, with the SE CoreValve Evolut R having incorporated an integrated sheathed delivery system and the BE SAPIEN 3 system with an atraumatic and smooth catheter profile with enhanced steerability.
Vascular Complications
The use of larger 22/24 F sheaths with the first generation BE SAPIEN valve was associated with a high rate of vascular complications as opposed to the smaller profile SE CoreValve.29 Recently, lower-profile BE valve systems such as the SAPIEN XT and SAPIEN 3 have become available with significant reduction in vascular complications. No significant difference between SE and BE groups with regard to vascular complications or bleeding was noted in the CHOICE trial (any vascular complication in 14 % for BE SAPIEN XT versus 12.8 % for SE CoreValve; p=0.78).20
Device Success
The composite endpoint of device success defined by the Valve Academic Research Consortium (VARC) evaluates acute device and procedural characteristics, and requires absence of prosthesis stenosis/regurgitation and correct implantation of only one valve for a successful procedure. In the CHOICE trial, the primary endpoint of device success was seen in 95.9 % of 121 patients treated with the BE device as compared with 77.5 % of 120 patients treated with the SE device (relative risk 1.24, 95 % CI 1.12–1.37).20 This difference in device success rates was largely driven by rates of more than mild valvular regurgitation, seen in 18.3 % of SE CoreValve patients versus 4.1 % of BE SAPIEN XT patients (p<0.001), and the more frequent need for implantation of a second valve in the SE CoreValve group, at 5.8 % versus 0.8 % (p=0.03).
Post-TAVI Aortic Regurgitation
Procedure-related incidence of aortic regurgitation, which includes transvalvular and paravalvular forms, is associated with increased short- and long-term mortality after TAVI and is a major limiting factor to its expanded use.18,19 Paravalvular aortic regurgitation results from poor annulus-prosthesis apposition and several factors, such as heavily calcified cusps, annulus-prosthesis mismatch and suboptimal prosthesis positioning, are thought to be responsible in both the SE and BE valve implantations. Transvalvular or central aortic regurgitation can be the result of leaflet restriction or damage during crimping or implantation as well as overdilatation of the valve.30
Some degree of aortic regurgitation, mostly mild, is observed in the majority of TAVI patients up to one-year follow-up. The incidence of more than mild aortic regurgitation after TAVI was found to be 7.4 % in a meta-analysis of post-TAVI outcomes.31 In another recent meta-analysis, the pooled estimate of more than mild post-TAVI aortic regurgitation was 11.7 % with a 95 % confidence interval of 9.6–14.1.32 In the UK TAVI registry, paravalvular regurgitation occurred more commonly in the SE CoreValve implants as compared with the BE SAPIEN implants (17.3 % versus 9.6 %).13 A similar finding was observed in the meta-analysis by Athappan et al.32 who reported more than mild aortic regurgitation to be more common with use of the SE CoreValve as compared with the BE valves (16.0 % vs. 9.1 %, p = 0.005). In the landmark CHOICE trial, the incidence of more than mild aortic regurgitation by angiography was 4.1 % in the BE group as compared with 18.3 % in the SE group (p<0.001).20 In fact, the primary endpoint in this trial was mainly driven by a significantly lower frequency of more than mild aortic regurgitation as well as a lower need for an additional device in the BE group. Implantation of the BE SAPIEN 3 valve resulted in no severe aortic regurgitation at 30 days of follow-up in a recently published trial, and the rate of more than mild aortic regurgitation was as low as 3.5 %.25
Sherif et al.33 investigated anatomic predictors of paravalvular regurgitation after SE CoreValve implantation and found the angle of the left ventricular outflow tract (LVOT) to the ascending aorta (AA) as well as the device depth in relation to the noncoronary cusp to be independent predictors. Paravalvular regurgitation was more likely in patients with greater LVOT-AA angle as a result of the incomplete sealing of the paravalvular space. Too low as well as too high implantations also facilitated regurgitation via the uncovered struts and the space between prosthesis and annulus respectively. Despite high implantation and adequate sizing employed in the CHOICE trial, higher rate of regurgitation was observed in the CoreValve group, raising concerns regarding inadequate radial strength of SE devices, which can be a contributing factor for regurgitation. Long-term studies are necessary to chart the progression of paravalvular regurgitation with time, since geometric remodelling of the annulus with a reduction in the degree of regurgitation may occur over time. Such reductions in severity of regurgitation may be of relevance for SE prostheses.