Differential Indications for TAVI
In the early days of TAVI, prosthesis selection was largely determined by the size of the aortic annulus and that of the iliofemoral arteries, with the SE CoreValve being favoured in patients with larger aortic annulus and smaller iliofemoral arteries. In recent times, annulus morphology has influenced valve selection and SE CoreValve, thought to have the ability to adapt to various annular and root anatomies, is preferred by some operators for patients with an asymmetric aortic annulus on multislice computed tomography. Operator experience with regard to volume of cases being performed and familiarity with the device is likely to play a significant role in prosthesis selection, with low-volume centres more likely to use only one device type for all patients. Nevertheless, availability of a wide size array of newer generation BE and SE prostheses have made TAVI feasible for a wide range of patients with complex anatomies.
Traditionally, bicuspid AS is considered as a contraindication for TAVI because of the increased risk of associated adverse aortic events such as progressive aortic dilatation with device migration, aortic dissections, and significant paravalvular regurgitation owing to more frequent elliptical shape and asymmetric annulus. As a consequence of technological improvements and experience, TAVI has been increasingly performed with reasonable success in selected patients with bicuspid aortic valve stenosis. Because of the potential complications mentioned above, these patients may be favoured to receive a repositionable SE valve prosthesis. Patients with minimal aortic valve calcification and predominant regurgitation are also likely to benefit from SE valve prostheses.
Finally, SE valves may have a beneficial role in patients undergoing valve-in-valve TAVI for small degenerated aortic bioprosthetic valves, where fewer gradients are achieved due to supra-annular valve function. On the other hand, BE valves could be used for valve-in-valve implantations for all four valve positions, namely tricuspid, pulmonary, mitral and aortic, whereas the SE CoreValve could only be used in the aortic position.
Newer Generation Devices
Since the first TAVI in 2002, a reasonable number of randomised trials and registries exist that provide valuable insights as to what is an optimal SE or BE prosthesis. An ideal BE prosthesis is one that has a low profile and allows optimal and accurate positioning within the native a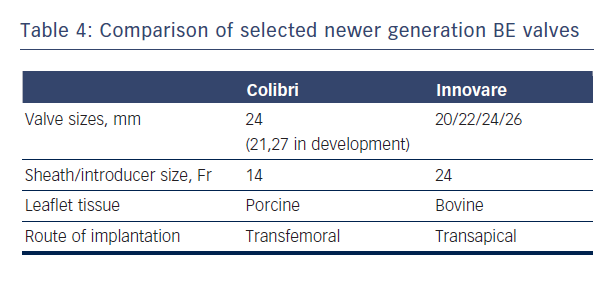
New generations of SE and BE devices address many of the major deficiencies of the first generation valves in terms of accuracy of positioning, repositionability, ease of use and enhanced features to minimise paravalvular leakage. Newer BE prostheses such as SAPIEN 3 and Colibri (Colibri Heart Valve, Broomfield, USA) and newer SE valves such as Evolute R and Lotus hold some promise in this regard.6
The Colibri valve is a low-profile (14-Fr) pre-crimped, and pre-packaged ready-for-use BE valve made of three independent pieces of porcine pericardium, which are created using a unique folding method and sewn into a stainless steel laser-cut frame. Another newer BE valve is the Innovare valve (Braile Biomedical, Brazil), which consists of a trileaflet bovine pericardial valve mounted in a cobalt-chromium alloy, available in sizes of 20/22/24/26 mm (see Table 4).43
Newer SE and mechanical valves such as Lotus (Boston Scientific, Natick, MA), Acurate (Symetis SA, Ecublens, Switzerland), Portico (St. Jude Medical, St Paul, MN), Centera (Edwards Lifesciences, Irvine, CA), Engager (Medtronic Minneapolis,MN) and JenaValve (JenaValve Technology GmbH, Munich, Germany) aim to address potential limitations of earlier SE devices, such as lack of repositionability and ease of use, and offer important advantages (see Table 5).44–48 For instance, the non-flared inflow end of the Portico valve permits higher implantation depth with minimal left ventricular outflow tract protrusion, thereby reducing the risk of heart block.45 Because the valve leaflets are fully functional in the partially deployed state, extent of paravalvular regurgitation can be assessed prior to full release. The next generation Lotus valve provides a high radial strength and is also capable of being completely retrieved if necessary.44 It has an adaptive seal at the inflow portion that serves to improve apposition to the native annulus and reduce paravalvular regurgitation.
Finally, the Direct Flow (Direct Flow Medical, Santa Rosa, CA) is a new distinctive prosthesis having a non-metallic valve frame and a flexible low-profile delivery system that allows repositionability and repeated assessment of full haemodynamic performance before final implantation. It consists of a bovine pericardial tissue valve that is mounted between two inflatable polyester rings and has the ability to adapt to the native aortic annulus and the left-ventricular outflow tract.49