Aortic Regurgitation and Indications for Valve Replacement
NAVR arises from failure of the aortic valve leaflets to appose during ventricular diastole, a process that may occur because of leaflet damage or distortion or from dilatation of the ascending root. Whereas a fibro-calcific degenerative process accounts for the large majority of cases of AS, the aetiologic spectrum of aortic incompetence is much broader. Globally, rheumatic heart disease accounts for a large burden of isolated aortic incompetence. In the developed world, degenerative and congenital conditions, such as bicuspid aortic valve, are more common causes of isolated aortic regurgitation.16 Enlargement of the aortic root and ascending aorta can occur concurrently and are frequently interdependent. The prevalence estimates of NAVR vary widely depending of the definition of aortic regurgitation used and the characteristics of the population: 2–30 %.16 The prevalence of significant NAVR in the general population is estimated at 1 %.16
Patients with mild aortic regurgitation rarely develop significant symptoms or LV dysfunction: even severe aortic regurgitation can remain indolent for a prolonged period of time. Symptoms may develop, however, once the imbalance between diastolic LV distension, myocardial muscle mass, and systolic wall tension leads to progressive LV dilatation and systolic dysfunction. Prognosis in patients with severe aortic regurgitation is associate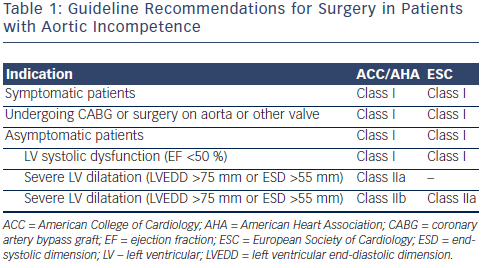 d with both the presence of symptoms and LV dysfunction. In a study of 246 patients with moderate of severe aortic regurgitation, 10-year mortality was closely associated with symptoms: those in New York Heart Association (NYHA) class II–IV had an annual mortality of 25 % compared with the 3 % mortality seen in asymptomatic patients.17 In addition, patients with LV dysfunction had a threefold greater mortality than those with preserved function.17 A long-term postoperative study has demonstrated improved survival when patients undergo early AVR after onset of mild symptoms, mild LV dysfunction (ejection fraction [EF] 45 % to 50 %), or end-systolic dimension 50 to 55 mm rather than waiting for more severe symptoms or more severe LV dysfunction to develop.18 Accordingly, the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines recommend aortic valve replacement when symptoms supervene, or once LV dilatation (LV end-systolic diameter >55 mm) or LV dysfunction (LVEF <50 %) develop (see Table 1).19
d with both the presence of symptoms and LV dysfunction. In a study of 246 patients with moderate of severe aortic regurgitation, 10-year mortality was closely associated with symptoms: those in New York Heart Association (NYHA) class II–IV had an annual mortality of 25 % compared with the 3 % mortality seen in asymptomatic patients.17 In addition, patients with LV dysfunction had a threefold greater mortality than those with preserved function.17 A long-term postoperative study has demonstrated improved survival when patients undergo early AVR after onset of mild symptoms, mild LV dysfunction (ejection fraction [EF] 45 % to 50 %), or end-systolic dimension 50 to 55 mm rather than waiting for more severe symptoms or more severe LV dysfunction to develop.18 Accordingly, the American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guidelines recommend aortic valve replacement when symptoms supervene, or once LV dilatation (LV end-systolic diameter >55 mm) or LV dysfunction (LVEF <50 %) develop (see Table 1).19
TAVR for Native Aortic Valve Regurgitation— Experience with the First-generation Transcatheter Heart Valves
Initial attempts at catheter-based treatment of NAVR in patients deemed surgically inoperable used first-generation transcatheter heart valves, such as the CoreValve and the Edwards SAPIEN valve. The CoreValve has hitherto been the preferred valve because of its technical and design characteristics. In particular, self-expandable valves, such as the CoreValve, offer high and permanent recoil forces that are thought to provide better stability in the noncalcified native aortic valve apparatus compared with the balloon-expandable transcatheter valves. Though neither valve was designed to treat this condition, successful cases have been reported.
Edwards SAPIEN
The Edwards SAPIEN heart valve system, which consists of a tri-leaflet bovine pericardial valve and a balloon-expandable, stainless steel support frame, has been used infrequently in the treatment of aortic regurgitation. One report describes the trans-apical deployment of a 29 mm valve via mini-thoracotomy and under cardiopulmonary bypass for a patient who developed severe aortic incompetence despite a structurally normal valve following LV assist device implantation.10 The procedure required substantial over-sizing (29 mm valve for a 21 mm annulus that would normally require a 23 mm valve). No residual aortic incompetence was noted, but no follow-up data were provided. Another case report documented procedural failure, with deployment of a 26 mm Edwards SAPIEN valve prosthesis in a patient with severe AR and an aortic annulus diameter of 26 mm, followed by valve dislocation soon after deployment and embolization into the left ventricle.20 A 29 mm CoreValve was then deployed successfully after retrieval of the embolized Edwards SAPIEN valve.
CoreValv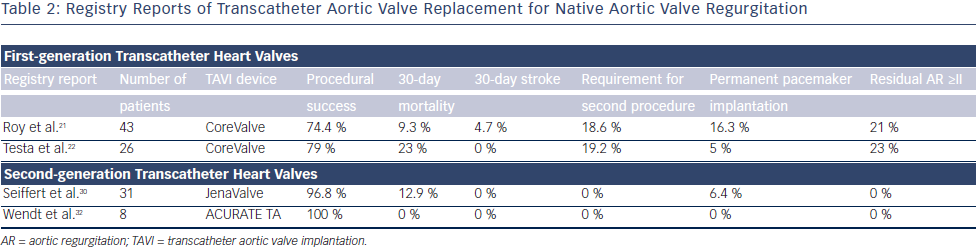 e
e
The CoreValve prosthesis consists of a tri-leaflet biological valve sewn into a self-expanding nitinol frame. An international registry study demonstrated the feasibility and potential procedural difficulties when using TAVI for severe AR21 (see Table 2). The study described 43 patients who underwent TAVR for NAVR with the CoreValve between 2011 and 2012 at 14 centers worldwide. All patients had pure severe AR and were deemed surgically inoperable with mean logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE), 26.9±17.9 %; and mean STS (Society of Thoracic Surgeons) score, 10.2±5.3 %. Although procedural success was high, with successful implantation of a TAVR in 42 out of 43 (97.7 %) patients (one conversion to open heart surgery), eight patients (18.6 %) required a second valve during the index procedure for residual aortic regurgitation. Post-procedure aortic regurgitation grade II or higher was present in nine patients (21 %), meaning that the Valve Academic Research Consortium (VARC)-defined procedure success was reduced to 74.4 %.21 At 30 days, the major stroke incidence was 4.7 %, and the allcause mortality rate was 9.3 %. At 12 months, the all-cause mortality was 21.4 % (six out of 28 patients). Also of interest was the finding that the need for a second valve was limited to patients without aortic valve calcification. This study demonstrated the feasibility of this technique and highlighted the potential procedural difficulties in treating NAVR with TAVR.
In a large CoreValve registry from Italy, 1,557 consecutive patients undergoing TAVR, of whom 26 (1.6 %) presented with AR, were prospectively followed and baseline clinical characteristics and clinical outcomes compared22 (see Table 2). Patients with AR were significantly younger (mean age 73±10 versus 82±6), more frequently suffered from advanced heart failure symptoms (NYHA Class III/IV 95 % versus 73 %), and had a higher incidence of severe pulmonary hypertension (SPAP >60 mmHg, 31 % versus 10 %) compared with patients with AS. Logistic EuroSCORE and STS scores were similar between the two groups and VARC-defined device success was lower in the AR group (79 % versus 96 %). At 1 month, patients treated for AR had a higher overall mortality (23 % versus 5.9 %, odds ratio [OR] 4.22) and cardiac mortality (15.3 % versus 4 %, OR 4.01). At 12 months, overall and cardiac mortality remained higher for patients who underwent TAVR for AR compared with AS (31 % versus 19 %, hazard ratio [HR] 2.1, and 19.2 % versus 6 %, HR 3.1, respectively).22