TAVR for Native Aortic Valve Regurgitation— Experience with the Newer-generation Transcatheter Heart Valves
A number of newer-generation transcatheter heart valves have been developed in recent years23 (see Table 2). Four devices that may be particularly suited for catheter-based treatment of aortic regurgitation are the JenaValve, ACURATE TA valve, the Medtronic Engager valve,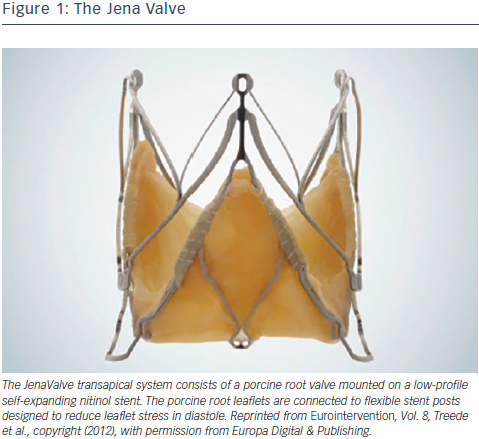 and the J-valve. In fact, the lack of calcification of the valvular apparatus confers greater stability to some newer-generation valves, and hence allows these devices to work more effectively. These valves share several characteristics. They are deployed primarily transapically; they are self-seating; they are anatomically oriented into the commissures; and they can be deployed in animal models in the orthotopic position because of their lower radial force compared with other TAVR devices.
and the J-valve. In fact, the lack of calcification of the valvular apparatus confers greater stability to some newer-generation valves, and hence allows these devices to work more effectively. These valves share several characteristics. They are deployed primarily transapically; they are self-seating; they are anatomically oriented into the commissures; and they can be deployed in animal models in the orthotopic position because of their lower radial force compared with other TAVR devices.
The JenaValve
The JenaValve transapical system consists of a porcine root valve mounted on a low-profile self-expanding nitinol stent24 (see Figure 1). The porcine root leaflets are connected to flexible stent posts designed to reduce leaflet stress in diastole. In contrast to devices expanding within the aortic annulus, the JenaValve relies on an active clip fixation of the native aortic valve leaflets, thereby reducing the radial force applied on surrounding cardiac and aortic structures24 (see Figure 2). This design also prevents coronary compromise by the native leaflets or stent struts, and does not interfere with future coronary intervention. Because the valve deploys in an anatomically aligned position, rapid pacing and balloon inflation is not required during implantation.
Several initial reports demonstrated feasibility and successful treatment of aortic regurgitation using the JenaValve.25–30 In the largest series of 31 patients, transapical TAVR with a Je naValve for the treatment of NAVR was performed in 31 patients in nine German centers.30 Average age at time of procedure was 74 years, and all patients were deemed at high risk for cardiac surgery, with an average logistic EuroSCORE of 23.6 %. Procedural success was achieved in 30 out of 31 cases, with one patient requiring a second valve implantation due to valve dislocation. Two patients underwent either surgical or further transcatheter valvular re-interventions because of infective endocarditis or for increasing paravalvular regurgitation. All-cause mortality was 12.9 % and 19.3 % at 30 days and 6 months, respectively. Cerebrovascular events did not occur during follow-up, and a significant improvement in NYHA class was observed and persisted up to 6 months. Post-procedural aortic regurgitation was none/trace in 28 of 31 and mild in three of 31 patients. At present, the JenaValve is the only transcatheter valve that has been CE-marked for treatment of both AS and aortic regurgitation.
naValve for the treatment of NAVR was performed in 31 patients in nine German centers.30 Average age at time of procedure was 74 years, and all patients were deemed at high risk for cardiac surgery, with an average logistic EuroSCORE of 23.6 %. Procedural success was achieved in 30 out of 31 cases, with one patient requiring a second valve implantation due to valve dislocation. Two patients underwent either surgical or further transcatheter valvular re-interventions because of infective endocarditis or for increasing paravalvular regurgitation. All-cause mortality was 12.9 % and 19.3 % at 30 days and 6 months, respectively. Cerebrovascular events did not occur during follow-up, and a significant improvement in NYHA class was observed and persisted up to 6 months. Post-procedural aortic regurgitation was none/trace in 28 of 31 and mild in three of 31 patients. At present, the JenaValve is the only transcatheter valve that has been CE-marked for treatment of both AS and aortic regurgitation.
ACURATE TA Valve
The ACURATE TA device (Symetis SA Ecublens, Switzerland) consists of a self-expandable nitinol stent, available in three sizes, which acts as an anchoring structure within the aortic annulus through its upper and lower crowns23,31,32 (see Figure 3). The ACURATE valve is designed to achieve precise anatomical fixation in the subcoronary and suprannular position. A three-leaflet porcine valve is fixed to the lower part of the nitinol stent. A double poly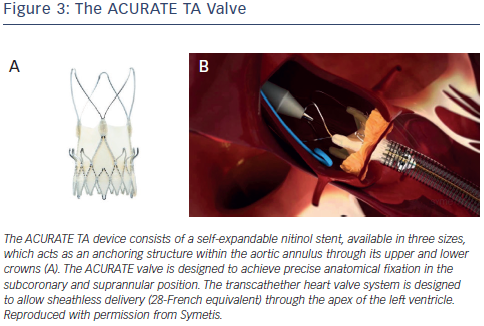 ethylene skirt covers the inner and outer surface of the stent body, thus reinforcing the porcine valve and avoiding direct contact between the biological tissue and the metal stent struts. The transcathether heart valve system is designed to allow sheathless delivery (28-French equivalent) through the apex of the left ventricle. Intra-procedural balloon valvuloplasty is generally not required for deployment, and implantation is carried out without rapid pacing.
ethylene skirt covers the inner and outer surface of the stent body, thus reinforcing the porcine valve and avoiding direct contact between the biological tissue and the metal stent struts. The transcathether heart valve system is designed to allow sheathless delivery (28-French equivalent) through the apex of the left ventricle. Intra-procedural balloon valvuloplasty is generally not required for deployment, and implantation is carried out without rapid pacing.
Wendt et al. describe procedural characteristics and clinical outcomes in a series of six patients with severe native aortic valve incompetence and two patients with aortic regurgitation secondary to failed aortic root repair, all of whom were deemed surgically inoperable in view of mean logistic EuroSCORE and STS scores of 34 % and 7.3 %, respectively.32 Procedural success was 100 % and no complications satisfying the VARC-2 criteria occurred in the peri-procedural period. Residual aortic incompetence post-procedure, as documented by transesophageal echocardiography, was at most grade I at three and six months’ follow-up. At 12 months’ follow-up, all-cause mortality, cardiovascular mortality, and cerebrovascular event rates were zero.32 Currently the ACURATE transcatheter valve is only available for transapical implantation, but a transfemoral version, the ACURATE T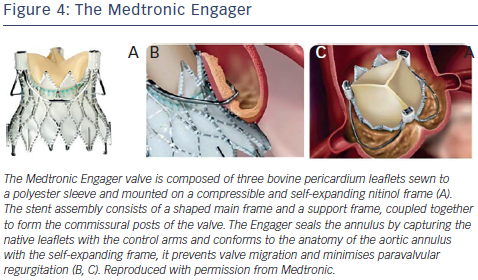 F, is undergoing clinical trials. Given the encouraging initial results, the ACURATE TA transcatheter heart valve might become more widely tested in the treatment of NAVR.
F, is undergoing clinical trials. Given the encouraging initial results, the ACURATE TA transcatheter heart valve might become more widely tested in the treatment of NAVR.
Medtronic Engager
The Medtronic Engager aortic valve system (Medtronic 3F Therapeutics, Inc., Santa Ana, California, US) is a second-generation TAVR bioprosthesis combined with a delivery system designed for over-the-wire transapical implantation of the valve. The valve is composed of three bovine pericardium leaflets sewn to a polyester sleeve and mounted on a compressible and self-expanding nitinol frame23,33 (see Figure 4). The stent assembly consists of a shaped main frame and a support frame, coupled together to form the commissural posts of the valve. The Medtronic Engager valve obtained CE (Conformité Européenne) mark in 2013, after the pivotal multicentre trial demonstrated feasibility and acceptable safety profile in 125 patients with inoperable AS.23
Because the Engager seals the annulus by capturing the native leaflets with the control arms and conforms to the anatomy of the aortic annulus with the se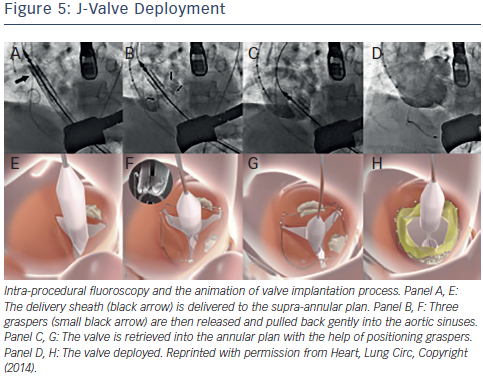 lf-expanding frame, it prevents valve migration and minimizes paravalvular regurgitation (see Figure 4). Although primarily intended for the treatment of severe AS, it has the potential to develop a role in the treatment of aortic regurgitation, given its unique technical design. Indeed, the Engager has been successfully used to treat noncalcific, severe aortic insufficiency in an inoperable patient with an excellent final echocardiographic result.34 However, the patient required permanent pacemaker implantation, a complication that occurred in a significant number of patients (28 %) recruited into the initial multicentre trial previously alluded to.33
lf-expanding frame, it prevents valve migration and minimizes paravalvular regurgitation (see Figure 4). Although primarily intended for the treatment of severe AS, it has the potential to develop a role in the treatment of aortic regurgitation, given its unique technical design. Indeed, the Engager has been successfully used to treat noncalcific, severe aortic insufficiency in an inoperable patient with an excellent final echocardiographic result.34 However, the patient required permanent pacemaker implantation, a complication that occurred in a significant number of patients (28 %) recruited into the initial multicentre trial previously alluded to.33
J-valve
The J-valve system (Jie-cheng Medical Technology, Suzhou, China) is a newly developed second-generation transcatheter heart valve designed for anterograde transapical implantation characterised by three U-shaped graspers that facilitate anatomically correct valve implantation and provide axial as well as radial fixation by embracing the native valve leaflets35 (see Figure 5). The first-inhuman implantation for a high-risk patient with severe NAVR was successful and excellent valve function was demonstrated at followup35 (see Figure 5).