Ancillary Devices
Helio Dock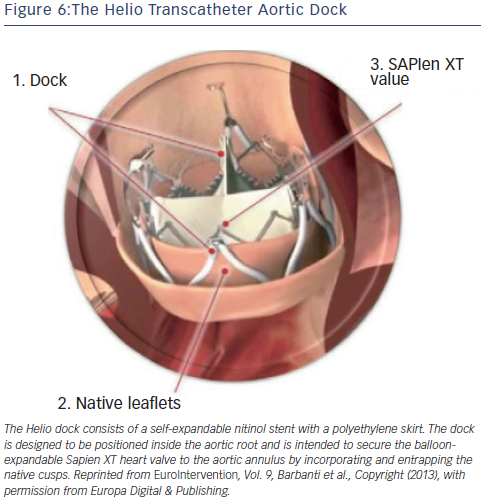
The Helio transcatheter dock (Edwards Lifesciences, Irvine, California, US) is an ancillary device that is intended to confer greater annular stability to the Edwards SAPIEN XT valve.15,36 The dock consists of a self-expandable nitinol stent with a polyethylene skirt (see Figure 6). The dock is designed to be positioned inside the aortic root and is intended to secure the balloon-expandable SAPIEN XT heart valve to the aortic annulus by incorporating and entrapping the native cusps. Following the first in-human successful report, a feasibility study of the procedure was demonstrated in a small series of four patients with severe primary AR deemed inoperable.37–39 Although initial results were encouraging, the dock technique has not gained traction, presumably due to the complexity of the technique and imminent availability of next-generation valves. At present, the manufacturer has discontinued production of the Helio dock.
Technical Considerations
Patient Selection
The use of TAVR for NAVR should still be limited to inoperable patients or those with prohibitive surgical risk due to age, frailty, or co-morbid conditions, as the clinical evidence accumulated thus far is not sufficient to establish noninferiority of TAVR compared with surgical treatment. From the Italian CoreValve registry, compared with inoperable patients with AS, patients who underwent TAVR for NAVR were younger, had more advanced NYHA class symptoms despite standard medical therapy, more frequently had SPAP, and had larger LV end-systolic as well as end-diastolic volumes. In the combined four largest series of TAVR for AR (a total of 108 patients), the predominant etiology of aortic incompetence was sclerotic-degenerative in 62 % of patients, followed by aortic aneurysm or dilatation in 13.9 % (see Table 3), with radiation therapy, infective endocarditis, rheumatic heart disease, vasculitis, and connective tissue diseases accounting for the remainder. The significant number of patients with aortic aneurysms is surprising, given that aortic regurgitation with concomitant aortopathy would normally mandate consideration of thoracic aortic surgical replacement with or without valve surgery.
Acute aortic in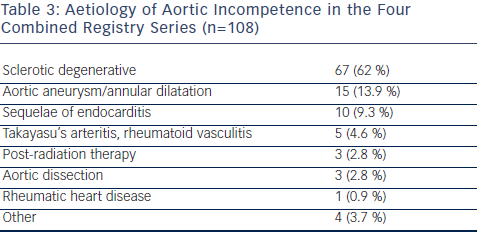 competence is generally attributable to dissection of the ascending aorta or infective endocarditis, and is not likely to be amenable to satisfactory transcatheter treatment.
competence is generally attributable to dissection of the ascending aorta or infective endocarditis, and is not likely to be amenable to satisfactory transcatheter treatment.
Diagnostic Work-up
Multimodality imaging, consisting of transthoracic and transesophageal echocardiography, and multidetector computed tomography imaging has been used increasingly in preparation for TAVR with the firstgeneration transcatheter valves, and is now accepted as standard practice. Contrast-enhanced multislice computed tomography is particularly useful for assessing valve and annular anatomy and aortic root morphology and should now be considered standard practice for planning TAVR procedures where available. Effective aortic annulus diameters, areas, and perimeters are often derived from multiplanar reconstruction of computed tomography data and/or from the midesophageal long-axis view in transesophageal echocardiography. In cases of NAVR, where precise valve dimensions are essential to prevent valve dislocation, multimodality imaging is essential.
Procedural Details
The newer-generation transcatheter valves are designed for implantation predominantly via the transapical route in the hybrid cardiac catheterization laboratory. Rapid or burst pacing is generally not required, as is balloon inflation and dilatation. Compared with TAVR for AS, TAVR for aortic incompetence has proved to be more technically challenging as reflected by the higher rate of valve-in-valve procedures required, and the lower rate of procedural success.
The absence of annular or cusp calcification translates into a lack of fluoroscopic markings that may make valve positioning more arduous compared to patients with calcific AS. The use of two pigtail catheters, the tips of which are positioned in the noncoronary and left coronary cusps may facilitate deployment.
Unresolved Matters and Future Directions
The experience in using the transcatheter approach to treat NAVR is limited but is expanding. Patients with mixed aortic valve disease with severe stenosis and at least moderate regurgitation have been successfully treated with both of the commercially available TAVR devices, but NAVR without stenosis is still considered a relative contraindication in published guidelines. Significant native aortic regurgitation is uncommon, and high-risk or inoperable patients with such pathology are few. Hence, the potential for this technology to expand exists, but is not of the same magnitude as with AS.
Newer-generation valve designs that use leaflet pinning or stabilization mechanisms are showing promise but experience with these devices is still limited. Larger registries, longer follow-up periods, and randomized clinical trials are necessary to gather sufficient evidence and experience to consider the widespread use of TAVR for NAVR.