Virtual Histology IVUS
Virtual histology (VH) IVUS is based on the spectral analysis of the raw backscattered IVUS RF data. It utilises a mathematical autoregressive model and constructs tissue maps that classify plaque into four major tissue types (fibrous, fibro-fatty, necrotic core, and dense calcium).5 VH-IVUS clinical utility ranges from predicting high-risk plaques for patients undergoing coronary angiography to predicting adverse events post percutaneous coronary intervention (PCI).6–9
Detection of High-risk Plaques
Rupture of an atherosclerotic plaque is the most common pathological substrate of acute myocardial infarction (AMI). Thin fibrous cap atheroma is a precursor to plaque rupture and is associated with sudden cardiac death.10 Although the axial resolution of VH-IVUS is too low to visualise thin fibrous cap <65 μm, it can potentially identify virtual histology thin cap fibroatheroma (VH-TFCA) that is defined as >10 % confluent necrotic core on three consecutive frames and arc of necrotic core in contact with the lumen for 36 degrees along lumen circumference.11 The prevalence of VF-TFCA is higher in acute coronary syndrome than in stable angina patients.12 In the Providing regional observations to study predictors of events in the coronary tree (PROSPECT) study, 697 patients presenting with acute coronary syndrome (ACS) underwent three-vessel grey-scale and VH-IVUS after successful PCI. The three-year cumulative rate of major adverse cardiovascular events (death from cardiac causes, cardiac arrest, myocardial infarction or rehospitalisation due to unstable or progressive angina) was 20.4 percent. Most events were rehospitalisation for unstable or progressive angina. Nearly half of these events (11.6 %) were related to non-culprit lesions.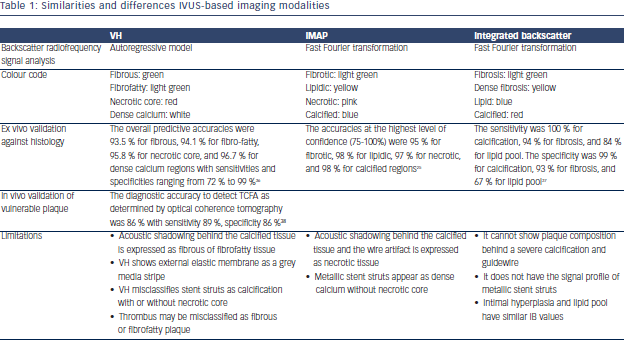
The PROSPECT study demonstrated that non-culprit lesions associated with recurrent events were frequently angiographically mild, most were VH-TFCA or were characterised by a minimal luminal area ≤ 4.0 mm2 or a plaque burden ≥ 70 %. Adverse events related to nonculprit lesions rarely developed from non-fibroatheromas, regardless of the minimal luminal area or plaque burden.6 Non-culprit lesions that had the non-fibroatheroma phenotype were associated with lower rate of future cardiovascular events than lesions with a fibroatheroma phenotype. We can speculate that VH-IVUS can predict lesion stability and defer intervention.7
In the Virtual Histology in Vulnerable Atherosclerosis (VIVA) study of 170 patients with stable angina or troponin positive ACS referred for PCI, VH-TCFA was associated with major adverse events defined as death, myocardial infarction and unplanned revascularisation.8 In line with previous studies, the European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis – Intravascular Ultrasound (ATHEROREMO-IVUS) study found that the presence of a VH-TFCA in a nonculprit coronary artery was independently predictive for the occurrence of major adverse cardiac events in stable and acute patients undergoing coronary angiography. VH-TFCA lesions were also independently associated with the composite of death or ACS only (present 7.5 % vs absent 3.0 %; adjusted hazard ratio (HR): 2.51, 95 % CI: 1.15–5.49; P = 0.021).9 Although VH-IVUS has been shown to predict major adverse cardiac events, it is unclear what treatment options might be effective in mitigating the risk associated with high-risk lesion features.
Use in Percutaneous Coronary Intervention
Grey-scale IVUS has been used to plan and guide PCI. Recently, few studies have tested clinical utility of VH-IVUS for guidance of PCI. The Bifurcation lesion analysis and stenting (BLAST) study investigated VH-IVUS guidance for drug-eluting stent deployment in bifurcation lesions compared to angiographic guidance alone enrolled 195 patients. Initial results showed that 30 days major adverse event rate was similar in both VH-IVUS unblinded and VH-IVUS blinded groups. Higher volumes of dense calcium and necrotic core at the side branch preintervention were contributing factors for major adverse events at 30 days.13 The long-term results have not been published yet. The Vascular evaluation for revascularisation: defining the indications for coronary therapy (VERDICT) study and Fractional flow reserve and intravascular ultrasound relationship study (FIRST) evaluated the prognostic utility of fractional flow reserve (FFR) and VH IVUS–derived parameters of atherosclerosis in intermediate coronary lesions (40–80 % angiographic diameter stenosis). There was only a modest correlation between IVUS minimum lumen area and FFR, but plaque morphology characteristics did not correlate with FFR.14,15
VH-IVUS can identify lesions that are at high-risk for causing distal embolisation or myocardial necrosis. Distal embolisation occurs in 15–70 % of patients and is associated with a poor prognosis after PCI. In a recent meta-analysis the association between plaque composition and distal embolisation after PCI was evaluated in 16 greyscale IVUS or VH-IVUS studies of 1697 patients. Pooled analysis showed that the absolute necrotic core volume, absolute and relative necrotic core areas at the minimum lumen sites were significantly greater in the embolisation group than in the no embolisation group. The other plaque components were similar in both groups.16 A previously published systematic review reported similar findings. In 9 of the 11 reviewed studies the necrotic core by itself or as a component of VH-TFCA was associated with distal embolisation.17 Two studies by Nakamura et al. and Bae et al. failed to demonstrate the association between necrotic core and distal embolisation, but a “marble”-like image, consisting of fibro-fatty and fibrous plaque, was associated 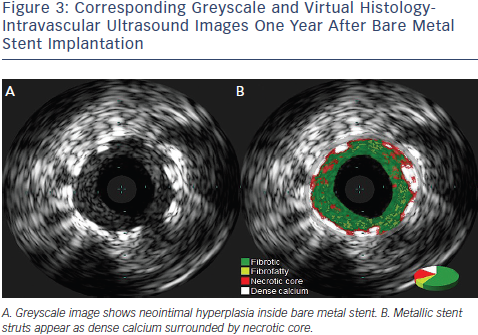 with the angiographic no-reflow phenomenon during primary PCI.18,19 This can be explained by the fact that thrombus by VH-IVUS has appearance of fibrotic or fibrofatty tissue and the percentage of necrotic core could be compromised by the presence of thrombus. In a study by Hong et al. in cohort of 80 patients with stable and unstable angina necrotic core volumes and areas were significantly greater in patients with post-PCI cardiac troponin I elevation.20 The use of statins and embolic protection devices could be particularly beneficial for patients with lesions with a large necrotic core. However no trial examining embolic protection devices in high embolic risk patients has yet been published. We also cannot conclude that VH-IVUS should be used routinely in all patients undergoing PCI.
with the angiographic no-reflow phenomenon during primary PCI.18,19 This can be explained by the fact that thrombus by VH-IVUS has appearance of fibrotic or fibrofatty tissue and the percentage of necrotic core could be compromised by the presence of thrombus. In a study by Hong et al. in cohort of 80 patients with stable and unstable angina necrotic core volumes and areas were significantly greater in patients with post-PCI cardiac troponin I elevation.20 The use of statins and embolic protection devices could be particularly beneficial for patients with lesions with a large necrotic core. However no trial examining embolic protection devices in high embolic risk patients has yet been published. We also cannot conclude that VH-IVUS should be used routinely in all patients undergoing PCI.
VH-IVUS has not been validated to assess plaque behind stents, but it has been used to evaluate the development of neoatherosclerosis within neointimal tissues after stent implantation (See Figure 3). VH-IVUS was performed in 36 lesions more than three years after stent implantation. In-stent VH-TFCA was identified in 45.5 % of bare metal stent-treated lesions and in 18.2 % after drug eluting stent implantation (p = 0.361). There was no statistically significant difference with regard to the in-stent tissue composition, including necrotic core and dense calcium.21
Effect of Pharmacological Intervention on Plaque Composition
VH-IVUS has also been used in several studies reporting serial changes of plaque composition in patients treated with lipid lowering therapies. However IVUS-VH acquisition is electrocardiogram-gated which makes accurate co-registration of serial studies difficult. Recently, in a substudy of the Study of coronary atheroma by intravascular ultrasound: the effect of rosuvastatin vs. Atorvastatin (SATURN) study serial VH-IVUS imaging was performed in 71 patients treated with rosuvastatin 40 mg or atorvastatin 80 mg daily for 24 months.22 This study showed a decrease in fibro-fatty tissue (P<0.001), while dense calcium increased (P=0.002). There were no changes in fibrous or necrotic core tissue volumes. However, these data are inconsistent with other studies. In meta-analysis by Tian et al. 17 studies involving 2171 patients receiving statin therapy were analysed, a regressive trend was reported for necrotic core volume (mean difference: –2.1 mm3; 95 % CI: –4.7–0.5 mm3, P = 0.11) while statin therapy did not induce a significant change for fibrotic, fibro-fatty, or dense calcium compositions.23 The Synergistic effect of combination therapy with cilostazol and probucol on plaque stabilization and lesion regression (SECURE) study compared the effects of nine months of treatment with probucol and cilostazol combination therapy or cilostazol monotherapy. This study failed to demonstrate significant differences in changes in plaque volume or composition between two groups despite different impacts on lipid biomarkers.24 However, there is no clear proof of a direct connection between changes in plaque composition and clinical events.