What is the MGuard Role in Primary PCI Today?
Dariusz Dudek
Dr Dariusz Dudek, Jagiellonian University (Krakow, Poland) began by presenting data that show that primary PCI caused a substantial decline of in-hospital mortality due to STEMI between 1960 and 2000.17 However, since then, STEMI mortality has plateaued at around 5 %, suggesting that additional strategies are required.18 The key goals of primary PCI are to restore epicardial flow; to prevent distal embolisation and no reflow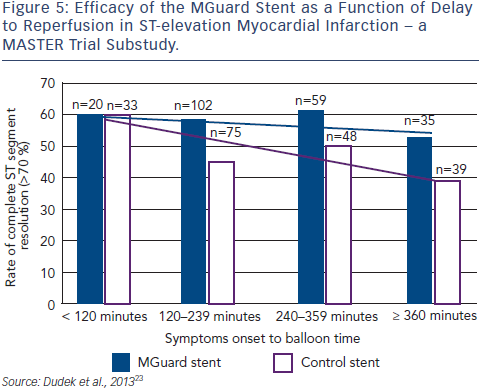 ; to improve myocardial reperfusion and to reduce infarct size. Recent studies suggest that DES are more beneficial than BMS in STEMI in terms of mortality.19 However, optimal microvascular/myocardial perfusion (TIMI 3 and myocardial blush 2/3) is often not achieved in PCI,8 with detrimental effects on longterm mortality.20
; to improve myocardial reperfusion and to reduce infarct size. Recent studies suggest that DES are more beneficial than BMS in STEMI in terms of mortality.19 However, optimal microvascular/myocardial perfusion (TIMI 3 and myocardial blush 2/3) is often not achieved in PCI,8 with detrimental effects on longterm mortality.20
Clinical data using the MGuard was first published in 2010.21 At that time, data from the MAGICAL trial in Krakow showed that the use of MGuard EPS implantation during primary PCI for STEMI is safe and is associated with excellent results in terms of myocardial reperfusion parameters. Importantly, no thrombus protrusion was seen with MGuard. The early safety and efficacy of the MGuard stent was maintained during the long-term follow-up (mean follow-up of 38.7 ± 3.1 months).22 In a substudy of the MASTER trial, patients were divided into two groups. Those with symptom onset to balloon time ≤3 hours when thrombus is fresh, and >3 hours. Later presentation of MI showed a bigger difference between MGuard and control in terms of rate of resolution of STEMI and percentage of patients achieving TIMI 3 flow than in earlier presentation, an effect that becomes more pronounced over time (see Figure 5).22
In conclusion, Dr Dudek recommended that use of a mesh covered stent should be an important component of the decision-making process when a STEMI patient arrives in the catheterisation laboratory and that a combination of a DES, bioresorbable vascular scaffold and mesh covered stent would be desirable.
Preliminary Results From the iMOS Prime Registry International MGuard Prime Observational Study – Acute and 30-day Results
Giovanni Amoroso
Dr Giovanni Amoroso, OLVG (The Netherlands) began by outlining the advantages of the MGuard Prime EPS, and then outlined a prospective, observational registry study whose objective was to evaluate the clinical performance of the MGuard Prime EPS in ‘real world’ STEMI patients undergoing primary PCI. Inclusion criteria was STEMI diagnosis with indication for primary PCI and reference vessel diameter of 2.75–4.0 mm. Exclusion criteria were cardiopulmonary resuscitation, cardiogenic shock, excessive tortuosity or calcification of the target vessel and side branches >2.0 mm. Baseline characteristics were similar to those in the MASTER trial,3 with baseline TIMI flow of 0/1 in 71.6 % of patients. The mean symptom-toballoon time was quite long: 237.8 min; most patients were preloaded with clopidogrel (94.8 %) and had undergone aspiration (74.2 %). Procedural success was 100%, without any problems of crossing the lesion or deploying the stent. TIMI flow of 3 was achieved in 91.8 % of patients and complete (>70 %) ST resolution in 76.1 %; these data are similar to those reported in the MASTER trial.3 At 30 days, there was a low incidence of MACE (2.2 %), without any death.
In conclusion, this is the first report investigating the use of the MGuard Prime EPS in ‘real world’ patients. Results show that the MGuard Prime EPS has a good safety and efficacy profile. Twelvemonth follow-up, to assess the impact on late MACE and clinical restenosis, is ongoing.
MASTER II – MGuard Prime Embolic Protection Stent
Jose Henriques
Dr Jose Henriques, Academic Medical Center, (Amsterdam, The Netherlands) presented the Safety and Efficacy Study of MGuard Stent After a Heart Attack II (MASTER II) study. This aims to include 1100 patients with acute MI for >30 minutes and ≤ 12h from symptom onset with >2 mm ST elevation in ≥2 contiguous leads on baseline ECG. Exclusion criteria include left bundle branch block (LBBB), paced rhythm or other ECG abnormality interfering with assessment of ST-segment; current enrolment in another clinical trial that may interfere with the current study endpoint; a previous coronary interventional procedure of any kind within 30 days prior to the procedure; female patients of childbearing potential; subjects undergoing cardiopulmonary resuscitation; cardiogenic shock; the anticipated need for a staged procedure of a target vessel within 12 months or non-target vessel within seven days post-procedure; prior administration of thrombolytic therapy; comorbid conditions that might affect patient compliance with follow-up; concurrent medical condition with a life expectancy of <12 months and a history of cerebral vascular accident or transient ischaemic attack within the last six months or permanent neurological deficit. A wide variety of stents may be used as control.
After coronary angiography, and TIMI 2/3 has been achieved, patients are randomised to the MGuard Prime EPS or BMS/DES. Clinical follow-up is at 30 days, six months and 12 months. Extended follow-up is at two and three years. The primary endpoints are the rate of complete ST-segment resolution (>70 % resolution of the sum of ST elevations in all leads) within 60–90 minutes post-procedure, as well as a composite of allcause mortality or recurrent target vessel MI at 365 days post-procedure.
Powered secondary endpoints include infarct size assessed by cardiac MRI in patients with anterior MI and proximal/mid LAD lesions, which is powered for superiority, and in-stent late lumen loss as measured by quantitative coronary angiography at 13 months; this is powered for non-inferiority.
The publication of this article was supported by InspireMD.