Percutaneous coronary intervention (PCI) involving stenting is routine practice, and involves either bare metal stents (BMS) or drug-eluting stents (DES), which allow controlled release of antiproliferative drugs at the arterial waazll.1 However, the persistence of durable polymers in first-generation DES led to numerous problems including inflammation, delayed arterial healing, aneurysm formation and mechanical disruption. All of which resulted in potential stent thrombosis.2 More recently, advances in technology have resulted in the emergence of DES with novel features to enhance efficacy and safety, including the Cre8™ polymer-free DES (Alvimedica). A satellite symposium, chaired by Dr Didier Carrié of Rangueil Hospital (Toulouse, France) and Marco Valgimigli of Erasmus Thoraxcenter (Rotterdam, The Netherlands), was held at EuroPCR on May 21st 2014 in Paris. The objectives of this symposium were to review clinical study data for the Cre8 DES, understand the unmet needs in DES clinical performance for PCI in everyday practice and to understand the added efficacy and safety value of the polymer DES technology.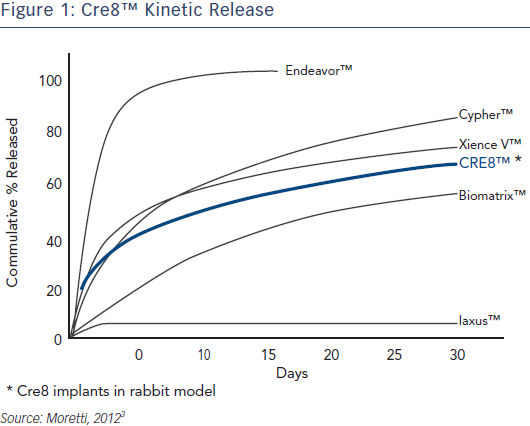
Dr Carrié began by introducing the Cre8 technology and reviewing published clinical data. The Cre8 DES uses a proprietary polymerfree drug release system (Abluminal Reservoir Technology), which comprises reservoirs on the outer surface of the stent. These enable controlled drug elution that is directed exclusively towards the vessel wall. The Cre8 DES utilises a formulation of sirolimus plus an organic acid, Amphilimus™, that enhances bioavailability and drug distribution to the entire vessel wall. Studies of a rabbit model show that the Cre8 DES has steady release kinetics compared to other commercially available DES (see Figure 1), reaching peak drug concentration during the first few days, 50 % drug elution in approximately 18 days, 65–75 % drug elution within 30 days and complete drug elution within 90 days.3 Its optimised permeability allows a homogeneous distribution inside the vessel wall and a uniform action on the whole tissue, enabling an optimal balance between safety and efficacy. After drug release, the Cre8 could be considered a bare metal stent (BMS). Cre8 is covered with a bio-inducer surface made of pure carbon, which is biocompatible and does not produce any late inflammatory stimuli inside the treated segment, reducing inflammatory response and lowering the risk of device thrombogenicity.
Clinical data in support of the efficacy of the Cre8 DES has been obtained from the NEXT clinical study, which enrolled 323 patients with ischaemic myocardial symptoms relat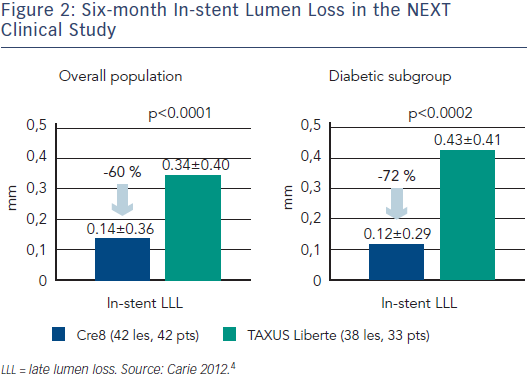 ed to de novo lesions in native coronary arteries, in 11 European sites.4 These were randomised 1:1 to the Cre8 or the Taxus Liberté stent. The primary endpoint was six-month angiographic in-stent late lumen loss (LLL). Although the trial was a non-inferiority trial, the Cre8 demonstrated superiority over the Taxus Liberté stent. In-stent LLL was significantly lower in the Cre8 group (0.14 mm vs 0.34 mm, p non-inferiority <0.0001, p superiority <0.0001). Clinical endpoints (cardiac death, myocardial infarction, target lesion revascularisation, and stent thrombosis) up to 12 months did not differ significantly between the two groups. The most surprising finding of this study was that the LLL in the diabetic subgroup was comparable to that obtained in the overall population, a finding that had not been seen before with DES (see Figure 2). Only one stent thrombosis was seen in each group. The study concluded that the Cre8 stent in de novo lesions showed significantly lower in-stent LLL at six months than the Taxus Liberté stent, with a trend toward better 12-month clinical safety and efficacy results.
ed to de novo lesions in native coronary arteries, in 11 European sites.4 These were randomised 1:1 to the Cre8 or the Taxus Liberté stent. The primary endpoint was six-month angiographic in-stent late lumen loss (LLL). Although the trial was a non-inferiority trial, the Cre8 demonstrated superiority over the Taxus Liberté stent. In-stent LLL was significantly lower in the Cre8 group (0.14 mm vs 0.34 mm, p non-inferiority <0.0001, p superiority <0.0001). Clinical endpoints (cardiac death, myocardial infarction, target lesion revascularisation, and stent thrombosis) up to 12 months did not differ significantly between the two groups. The most surprising finding of this study was that the LLL in the diabetic subgroup was comparable to that obtained in the overall population, a finding that had not been seen before with DES (see Figure 2). Only one stent thrombosis was seen in each group. The study concluded that the Cre8 stent in de novo lesions showed significantly lower in-stent LLL at six months than the Taxus Liberté stent, with a trend toward better 12-month clinical safety and efficacy results.
Dr Carrié ended his presentation by stating that the Cre8 unique features and initial clinical results have identified it as a possible step forward in DES development for both safety and efficacy.
The publication of the article was supported by Alvimedica.