PCI in Everyday Clinical Practice: What is Still Unmet in DES Performance?
Dr Marco Valgimigli of the Erasmus MC Thoraxcenter in Rotterdam, the Netherlands, began by summarising the unmet needs in DES performance. These were long-term outcomes, performance in patients with diabetes mellitus and requirement for DAPT. An observational study comparing the effectiveness of revascularisation strategies found that, among older patients with multivessel coronary disease that did not require emergency treatment, there was an initial survival advantage among patients who underwent PCI as compared with patients who underwent coronary artery bypass grafting (CABG), but in the long-tem, the advantage switches to CABG.13 The more severe the coronary disease, the worse is the comparative effect of PCI. The results of the SYNTAX trial also showed an initial survival advantage with PCI, as long as the anatomy is not too complex.1 The FREEDOM trial reported that irrespective of complexity, CABG is superior to PCI in diabetic patients.14 However, stent technology has improved since these studies were undertaken. The SPIRIT IV trial demonstrated the benefits of the second generation DES, although all the advantages of the Xience™ stent in the first year were associated with non-diabetic patients.15 The EXCEL trial aimed to provide important information on whether the use of second-generation DES would make PCI more competitive in comparison to CABG in a complex patient population. In 2014, enrolment in the EXCEL trial was however capped at 1900 rather than the planned 2600. No other randomised studies are currently planned or ongoing with respect to the performance of second generation DES as compared to CABG. Hence, a full understanding how newer generation DES may fill the gap to CABG in current practice, remains unmet.
In terms of DAPT requirement the general consensus is that patients receiving DES should take DAPT for a minimum of 6/12 months or even more. This represents a significant disadvantage in an increasingly elderly and frail patient population, for whom the bleeding risk is at least as high as the ischaemic risk. The current treatment paradigm is BMS + one month of DAPT or DES + long-term DAPT. However, there is no evidence from major clinical studies to support DAPT duration of more than 12 months following DES implantation.16–19 Current guidelines suggest that patients at high bleeding risk should be given a BMS followed by DAPT for 30 days. However, a safe DES followed by DAPT of short duration may be a better option. The ZEUS study, which randomised patients to a zotarolimus-eluting stent or a BMS, suggested that DAPT duration should be personalised. For example, modelled according to the patient’s clinical risk profile and not by stent type. The study found a higher risk of MI in the BMS group. A post-hoc analysis found that these MIs were largely type 1 (spontaneous MI) but also type 4b (stent thrombosis), illustrating the power of late loss inhibition in a high-risk population. Studies have demonstrated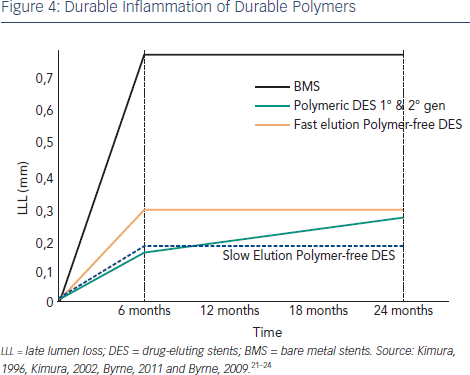 that the most important predictor of late stent thrombosis is the RUTTS score. Data from the Demonstr8 trial have shown that the Cre8 DES is associated with a very high percentage of struts with a RUTTS score <30 % (99.78 % of patients) indicating very good stent coverage. Hence, studies designed to prove the safety of a reduced DAPT duration after Cre8 implantation would be welcome to further understand if DAPT duration should be driven by patients’ characteristics and not stent type as long as new generation technology DES is employed.
that the most important predictor of late stent thrombosis is the RUTTS score. Data from the Demonstr8 trial have shown that the Cre8 DES is associated with a very high percentage of struts with a RUTTS score <30 % (99.78 % of patients) indicating very good stent coverage. Hence, studies designed to prove the safety of a reduced DAPT duration after Cre8 implantation would be welcome to further understand if DAPT duration should be driven by patients’ characteristics and not stent type as long as new generation technology DES is employed.
In conclusion, what is unmet in DES technology is our capability to prove their value under current market conditions. There is a need for studies looking at long-term outcomes, especially in patients with diabetes. There is also a need for studies comparing the efficacy and safety of current DES with that of CABG. In addition, we need to prove that DES efficacy does not necessarily mean longer duration of DAPT. The duration of DAPT should be tailored to the patient and not to the stent.
Cre8 in Diabetic Patients
Dr Rafael Romaguera of the University of Barcelona (Spain) discussed the question of whether the Cre8 stent can make a difference to patients with diabetes. He began by examining the causes of DES failure in diabetic patients. Compared with control smooth muscle cells, those cultured under high glucose conditions require more than 10x as much sirolimus concentration to achieve similar suppression.20 Differences in vascular c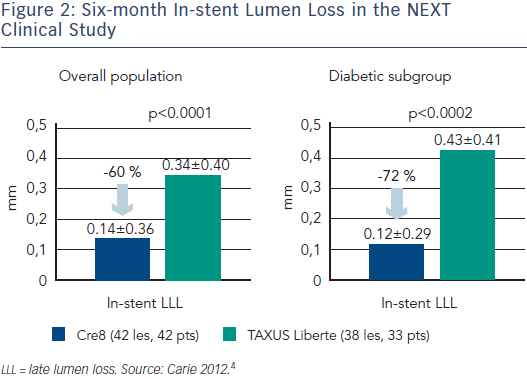 ell metabolism are also seen between diabetics and non-diabetics. In a basal setting, glucose and lactate account for approximately 30 % of energy, whereas 70 % of adenosine triphosphate (ATP) generation is derived from fatty acid oxidation. However, in diabetes, as glucose uptake and oxidation are impaired, the heart is coerced to use fatty acid almost exclusively for ATP generation. The Amphilimus™ technology employed in the Cre8 DES, in which the immunosuppressive drug is formulated with a long chain fatty acid, may enhance drug concentration, homogeneity and stability into the vascular cell.
ell metabolism are also seen between diabetics and non-diabetics. In a basal setting, glucose and lactate account for approximately 30 % of energy, whereas 70 % of adenosine triphosphate (ATP) generation is derived from fatty acid oxidation. However, in diabetes, as glucose uptake and oxidation are impaired, the heart is coerced to use fatty acid almost exclusively for ATP generation. The Amphilimus™ technology employed in the Cre8 DES, in which the immunosuppressive drug is formulated with a long chain fatty acid, may enhance drug concentration, homogeneity and stability into the vascular cell.
While BMS show a characteristic luminal response involving a rapid increase in LLL over the first six months, due to inflammation and followed by a levelling out,21,22 the first and second generation DES showed persistent inflammation and a continued increase in LLL after six months (see Figure 4).23,24 Theoretically, the Amphilimus™ formulation in the Cre8 DES may represent an advantage in diabetic patients with increased fatty acid metabolism. The absence of polymer may also avoid late events related to persistent inflammation. This has been demonstrated in the clinical trial setting, where the LLL in diabetes subgroup was comparable to that obtained in the general population (see Figure 2).4
The Randomised trial comparing reservoir-based polymer-free amphilimus‐eluting stents vs everolimus-eluting stents with durable polymer in patients with diabetes mellitus (RESERVOIR) trial is a prospective, randomised-controlled, single-blind, two-arm, multicentre clinical evaluation that aims to compare the Cre8 Stent implantation to polymer-based everolimus-eluting stent in diabetic patients (n=112). The primary endpoint is neointimal hyperplasia as determined using OCT. Secondary endpoints include percentage of uncovered struts, percentage of malapposed struts, maximum areas of obstruction and angiographic LLL.25
In summary, the PCI of patients with diabetes remains challenging, even with the second generation of DES. The Cre8 has demonstrated promising efficacy in patients with diabetes; however, the results need to be confirmed by the ongoing RESERVOIR trial, as well as future clinical trials.
The publication of the article was supported by Alvimedica.