Clinical Programme Update
Dr Gennaro Sardella of the University of Rome (Italy) presented an update on the ongoing studies in the Cre8 clinical program, beginning with an overview of DES development. The first-generation BMS were suboptimal in terms of both efficacy and safety. The secondgeneration BMS had improved safety profiles but little improvement in efficacy was seen. The advent of the DES resulted in a substantial improvement in efficacy, but first generations of DES had safety issues. Now, with the emergence of newer DES, an optimal balance of efficacy and safety is being achieved, although efficacy in diabetics remains an unmet need.
The features of the Cre8 DES that enhance its safety are the polymerfree platform, which avoids all the established drawbacks associated with the presence of polymer interface with blood flow or vessel wall; the bio-inducer surface that ensures optimal haemo-compatability vs lumen blood flow, and an abluminal reservoir that controls and directs elution to the vessel wall. The polymer-free platform, together with the amphilimus formulation of sirolimus and organic acid, enhancing drug bioavailability and permeability, contribute to the superior efficacy of Cre8.
Following the demonstration of efficacy and safety in the NEXT clinical trial, the next steps in the Cre8 clinical development program are a randomised clinical trial, Demonstr8, and a real-world study in the diabetic population, Prove Abluminal Reservoir Technology Clinical benefit in all comers patients (PARTicip8).
The Demonstr8 study
The rationale for the Randomised comparison between a DES and BMS to assess neointimal coverage by optical coherence tomography (OCT) examination (Demonstr8) study5 was that millions of stable patients undergoing PCI with BMS implantation have taken one-month dual antiplatelet therapy (DAPT), followed by aspirin monotherapy to optimise safety and efficacy of PCI procedure according to European guidelines. A longer duration of DAPT is required with DES use, since incomplete endothelial stent strut coverage and malapposition is considered a predictor of stent thrombosis. Fu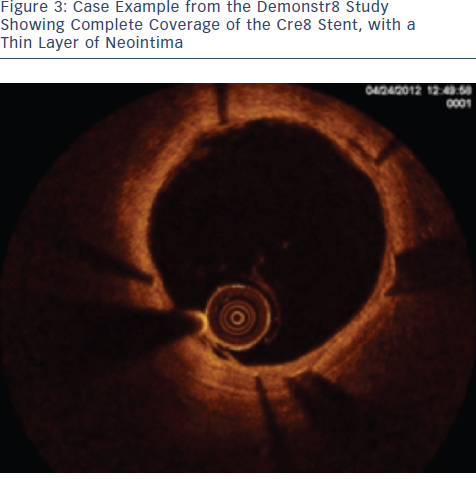 rthermore, heterogeneity of healing is commonly seen in DES.6 However, after complete drug elution, the Cre8 becomes a BMS and interacts with blood and tissue as a standard BMS. The Demonstr8 study therefore aims to show non-inferiority of the Cre8 in terms of stent strut coverage evaluated with optical coherence tomography (OCT) at three months after stent implantation compared with a well known BMS (Vision Multilink).7 It has been hypothesised that if endothelial coverage is comparable at three months, the Cre8 could be treated as a BMS at this stage; i.e. only aspirin would subsequently be needed.
rthermore, heterogeneity of healing is commonly seen in DES.6 However, after complete drug elution, the Cre8 becomes a BMS and interacts with blood and tissue as a standard BMS. The Demonstr8 study therefore aims to show non-inferiority of the Cre8 in terms of stent strut coverage evaluated with optical coherence tomography (OCT) at three months after stent implantation compared with a well known BMS (Vision Multilink).7 It has been hypothesised that if endothelial coverage is comparable at three months, the Cre8 could be treated as a BMS at this stage; i.e. only aspirin would subsequently be needed.
The study recruited 38 patients with ischaemic myocardial symptoms related to de novo lesions in native coronary arteries, in six European sites.5 The primary endpoint was the percentage of sections with a Ratio of Uncovered to Total Stent Struts per Cross Section (RUTTS) score < 30 % at three-month non-inferior to Vision Multilink percentage of sections with RUTTS score < 30 % at one month. The 35 patients suitable for analysis have led to the evaluation of 17,000 struts in 2000 analysed sections. RUTTS scores <30 % were seen in 99.78 % of patients receiving a Cre8 DES and 99.55 % of patients receiving a BMS. In terms of secondary endpoints, OCT analysis showed superiority of the Cre8 group in terms of mean neoimtima thickness at months one and three (0.08±0.03 mm in Cre8 group vs 0.18±0.10 mm in BMS group; p<0.0001; see Figure 3). In conclusion, results to date from this study show that Cre8 has an excellent safety profile, with low RUTTS scores and low neointima thickness.
The PARTicip8 Trial
The PARTicip8 clinical observational prospective study aims to involve around 1000 ‘real world’ patients with ischaemic myocardial symptoms related to de novo lesions in native coronary arteries, in 30 European sites.8 One hundred patients from a pre-specified diabetic subgroup will be submitted to angiographic follow up. The primary endpoint is a composite of cardiac death, target vessel myocardial infarction (MI) and clinically indicated TLR at six months. The recruitment was closed in December 2013 with an increased number of patients from the initial plan – 1250 patients.
The Investig8 Trial
The Multicentric and retrospective registry in real-world patients with polymer-free drug eluting stent Cre8 (INVESTIG8) study aims to collect clinical evidence of Cre8 performance from around 1,000 patients in a maximum of 15 European centres. The primary endpoint is the incidence of a composite of cardiac death, target vessel MI and clinically indicated target lesion revascularisation (TLR) at 12 months (major adverse cardiac events (MACE) at 12 months); secondary endpoints are the incidence of a composite of all deaths, all MI and any revascularisation at 12 months, as well as the incidence of stent thrombosis.
Interim analysis of data from 346 patients showed that 34.68 % of the patients had diabetes and 90.8 % had target lesions classified B2 or C according to the American College of Cardiology (ACC)/ American Heart Association (AHA) classification. The results to date are extremely positive with MACE in only 4.6 %
In conclusion, available data from ongoing clinical studies show that Cre8 has excellent efficacy, without compromising safety, for a broad range of patients.
Clinical Programme Update
The Tel Aviv Medical Center Cre8 Study
Dr Shmuel Banai of the Tel Aviv Medical Center Israel began by summarising the current limitations of DES: the risk of late stent thrombosis and the inferior efficacy in patients with diabetes. In addition, non-homogeneous coverage of the metal struts due to breaks and cracks of the polymer may lead to inflammatory reactions in the vessel wall, promoting instant stent restenosis (ISR), as well as platelet reactivation, leading to stent thrombosis. Inflammation plays a key role in coronary development and progression in diabetic patients.9,10 In scanning electron microscopy (SEM) studies, inhomogeneous distribution of coating was recognised in all DES types examined.11 Furthermore, balloon expansion of first and second generation DES disturbs the polymer surface and can cause detachment of microparticles.12 The clinical implication of damaged polymers may have been under-recognised and may have a substantial impact on clinical outcomes of patients receiving these stents, especially in diabetic patients. Polymer-free DES may overcome these limitations.
The TLVMC Cre8 study was a prospective, single arm open label nonrandomised single-centre study and aimed to evaluate the safety and efficacy of the Cre8 stent in the all-comer population. The end points were death, MI, stroke, unplanned PCI and clinically driven TLR at 30 days, six and 12 months. Between Nov 2012 and Aug 2013, 215 patients were enrolled and 319 stents implanted. One-year follow-up data are available on all 215 patients. Five patients were diagnosed as non-ST elevation MI (NSTEMI), only one of which had focal in-stent restenosis in an right coronary artery (RCA) in which four Cre8 DES were implanted; in the other four patients, the non- STEMI events were related to a new lesion in a different coronary artery. A total of nine patients underwent clinically indicated PCI. Only one required TLR, in which the lesion was successfully treated with a drug-eluting balloon. Among the remaining eight, none required PCI to the index coronary artery. These data demonstrate a very low incidence of MACE, suggesting an excellent safety profile. The very low incidence of clinically driven TLR also suggests high clinical efficacy.
Dr Banai concluded by stating that polymer-free DES appear to represent a new and improved generation of DES, but that this needs to be confirmed by larger clinical trials and extended clinical experience.
The publication of the article was supported by Alvimedica.