Section C
Use of Adjunctive Devices and intravascular multislice tomography
Intravascular ultrasonography can facilitate the recanalisation of stumpless CTO lesions by revealing the entry point of the occlusion when the occlusion site is angiographically unrecognisable due to its smooth entrance into the adjacent side branch. Moreover, intravascular ultrasonography can facilitate the repositioning of a guidewire in the event of an inadvertent subintimal passage and enable the selection of an optimal balloon size when reverse CART is performed.38
Multislice computed tomography can provide a detailed characterisation of the amount of calcification, tortuosity and length of the occluded segment, which can facilitate the procedural planning and estimation of the probability of successful PCI. Moreover, 3D reconstruction of the coronary anatomy and its integration with 2D fluoroscopy images during CTO PCI may help identify the best angiographic projection and provide a directional guide for the “missing segment” in the angiography.49
Section D
CTO PCI Complications
Complications during CTO PCI can be either acute or occur during long-term follow-up. Acute CTO PCI complications can be classified as coronary artery-related, (such as coronary occlusion, coronary perforation, and equipment loss or entrapment); cardiac non-coronary (such as periprocedural myocardial infarction, arrhythmias, and tamponade); and non-cardiac (such as vascular access complications, systemic embolisation, contrast-induced nephropathy, allergic reactions, and radiation-induced injury).16 A number of complications (such as donor vessel dissection or thrombosis) are specific to CTO interventions.16
A meta-analysis of 65 studies with 18,061 patients undergoing CTO PCI reported a low incidence of acute complications. These were death 0.2 % (95 % CI: 0.1 % to 0.3 %); emergent coronary artery bypass grafting 0.1 % (95 % CI: 0.0 % to 0.2 %); stroke <0.01 % (95 % CI: 0.0 % to 0.1 %); MI 2.5 % (95 % CI: 1.9 % to 3.0 %); Q-wave MI 0.2 % (95 % CI: 0.1 % to 0.3 %); coronary perforation 2.9 % (95 % CI: 2.2 % to 3.6 %); tamponade 0.3 % (95 % CI: 0.2 % to 0.5 %); and contrast nephropathy 3.8 % (95 % CI: 2.4 % to 5.3 %).50 Given the low frequency of emergency coronary artery bypass grafting, the authors believe that surgical backup is not essential for CTO PCI programs, provided there is a tested plan for urgent transfer to a facility with cardiac surgery in case of complications. However, the performance of CTO PCI at facilities without on-site surgery is discouraged unless performed by an operator with considerable experience in CTO PCI and at a laboratory that has immediately available all interventional equipment needed for CTO PCI and for the management of potential complications.
Although coronary perforations are common in CTO PCI (27.6 % in one series15), most perforations are related to localised wire exit sites from the vessel architecture and limited to angiographic evidence of contrast staining. In the above meta-analysis of CTO complications the risk of perforation was 2.9 % but the risk of tamponade only 0.3 %.50 There are three main perforation types. The first is main vessel perforation, which may require implantation of a covered stent. The second is distal wire perforation and the third is collateral vessel perforation, which may require coil e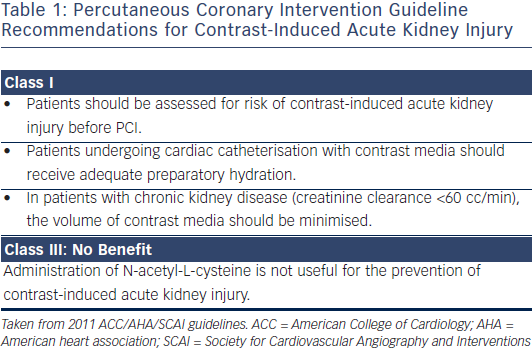 mbolisation.16 The availability of equipment for perforation management (covered stents and coils) and familiarity with their use is important for every CTO PCI program.
mbolisation.16 The availability of equipment for perforation management (covered stents and coils) and familiarity with their use is important for every CTO PCI program.
Contrast induced nephropathy (CIN), which was recently termed contrast induced acute renal injury (CI-ARI), is a well-recognised complication of invasive procedures using iodinated contrast agents. Patients at highest risk are those with baseline impaired renal function and diabetes. Mehran et al. developed a risk score for development of contrast nephropathy that includes eight variables (hypotension, intra-aortic balloon pump, congestive heart failure, chronic kidney disease, diabetes, age >75 years, anaemia and volume of contrast).51 CIN/CI-ARI is a contrast-dose dependent phenomenon.52 Laskey et al. determined that a contrast volume to creatinine clearance ratio >3.7 significantly increased the risk of CIN/CI-ARI in PCI patients.53 The use of this ratio to identify patients undergoing PCI who will benefit from less contrast and closer follow-up may improve care. Table 1 lists the recent 2011 ACC/AHA/ SCAI PCI guidelines for CIN/CI-ARI.41
Over the long-term, CTO interventions can be complicated by in-stent restenosis, stent thrombosis and coronary aneurysm formation. The use of drug-eluting stents significantly reduces the risk of restenosis (as described in part 1) without increasing the risk of stent thrombosis.54 The risk of coronary aneurysm formation and their management has been poorly studied. Among 560 patients undergoing CTO PCI in Japan, aneurysms were observed in 7.3 % of those where retrograde intervention was performed vs 2.6 % of those where antegrade intervention was performed.55 There is limited information on the long-term outcomes of the novel dissection/ re-entry and retrograde techniques.20 Target lesion revascularisation was required at five months for 52 % of 31 patients treated with the STAR technique,18 whereas target vessel revascularisation was required in 47.9 % of 74 patients treated with the contrast-guided STAR technique.56 In a single-centre study of 170 patients undergoing CTO PCI patients in whom the CrossBoss and Stingray devices were used (n=60) had similar long-term outcomes with 110 p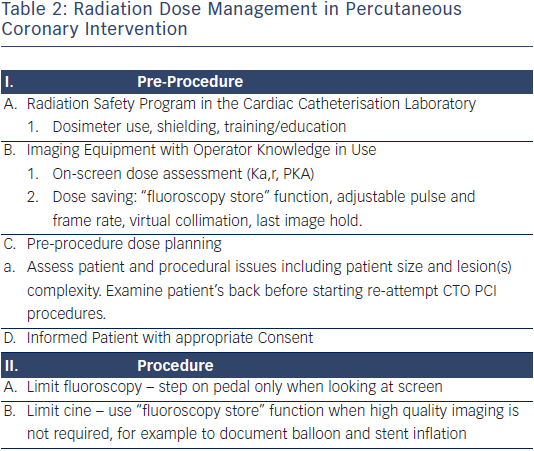 atients treated with other crossing strategies, in spite of its use in higher complexity cases.57 There are no published, long-term, outcome data on the use of the mini-STAR and LAST techniques (20). Similarly, only one of the 20 studies that examined the outcomes of patients treated with the retrograde approach provided long-term outcomes.20 The incidence of major adverse cardiac events in 24 patients during a median follow-up of 10.3 months was 18 %.58 Obtaining additional long-term data will be critical for assessing the comparative effectiveness of the various CTO crossing strategies.
atients treated with other crossing strategies, in spite of its use in higher complexity cases.57 There are no published, long-term, outcome data on the use of the mini-STAR and LAST techniques (20). Similarly, only one of the 20 studies that examined the outcomes of patients treated with the retrograde approach provided long-term outcomes.20 The incidence of major adverse cardiac events in 24 patients during a median follow-up of 10.3 months was 18 %.58 Obtaining additional long-term data will be critical for assessing the comparative effectiveness of the various CTO crossing strategies.
Given that CTO PCI may expose patients to significant amounts of radiation, those patient should undergo careful follow-up to diagnose radiation-induced skin injury.59 The fluoroscopy time should not be used as the sole method for measuring radiation exposure because fluoroscopy time does not include cine imaging. Therefore, fluoroscopy time in and of itself is not a useful descriptor of the patient radiation dose. Total air kerma at the interventional reference point (Ka,r, Gy) is the procedural cumulative air kerma (X-ray energy delivered to a volume of air) at the interventional reference point and is used to monitor deterministic skin effects. No observable effects are present with Ka,r, <2 Gy while effects may occur at >5 Gy, with significant skin injury possible at >15 Gy. In clinical practice, much higher doses could be tolerated before radiation skin injury occurs if multiple imaging angles are used, as this would produce smaller, single-site, peak skin doses than would occur if the X-ray source were stationary. Table 2 outlines a comprehensive approach to patient dose management that should be standard practice in all interventional laboratories, especially those performing CTO PCI.59
The authors would like to thank Ms Sheila Agyeman for her invaluable effort in coordinating the manuscript creation process.