Left Ventricular Outflow Tract Obstruction
LVOTO at rest is encountered in ~30 % of patients with HCM, and is associated with limiting symptoms (dyspnoea, angin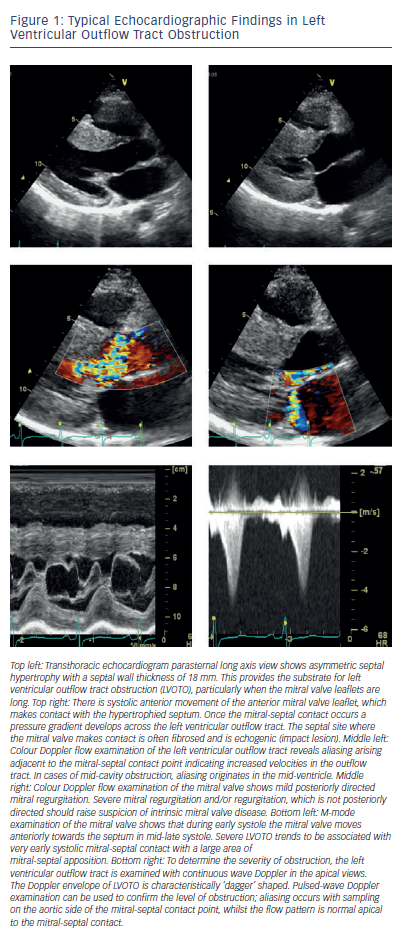 a, syncope) and worse prognosis.4–8 Unlike symptom limitation from ischaemic heart disease and left ventricular systolic dysfunction, effort tolerance in obstructive HCM is often variable, and patients often describe both good and bad days; this may make assessments of functional class (for example using the New York Heart Association [NYHA] classification) challenging. Approximately a third of HCM patients report postprandial exacerbation of symptoms.28
a, syncope) and worse prognosis.4–8 Unlike symptom limitation from ischaemic heart disease and left ventricular systolic dysfunction, effort tolerance in obstructive HCM is often variable, and patients often describe both good and bad days; this may make assessments of functional class (for example using the New York Heart Association [NYHA] classification) challenging. Approximately a third of HCM patients report postprandial exacerbation of symptoms.28
LVOTO is caused by the SAM of the mitral valve. In systole, the anterior mitral valve leaflet moves into the left ventricular outflow tract (LVOT), which is already narrowed by the hypertrophied septum creating a physical barrier, which impedes the flow of blood from the ventricle to the aorta during systole (see Figure 1).2,3 Contact of the mitral valve leaflets to the septum is termed ‘complete’ SAM, and lesser forms of SAM where there is no contact are termed ‘incomplete’. SAM of the mitral valve leaflets is often associated with varying degrees of mitral regurgitation since the two mitral valve leaflets are pulled apart during SAM, creating an orifice through which retrograde, posteriorly-directed flow in the left atrium can occur. Consequently, conditions which increase LVOTO also increase the severity of mitral regurgitation.3,29 LVOTO is also promoted by coincident abnormalities of the mitral valve leaflets. The leaflets (particularly the anterior leaflet) are frequently elongated and displaced anteriorly, with abnormal attachments to the papillary muscles and chordae.30,31 Early investigators attributed SAM of the mitral valve to suction forces (Venturi effect) caused by accelerating blood flow in LVOT during systole, drawing the mitral valve leaflets anteriorly into the outflow tract. However, SAM of the mitral valve commences in early systole when the Venturi effect is minimal, and this is therefore unlikely to be the sole explanation for LVOTO.32,33 The mechanism underlying LVOTO is probably multi-factorial, involving the interaction of abnormalities of papillary muscles, chordae, mitral leaflets and LVH, which culminate in abnormal flow forces that push and/or pull the mitral valve towards the outflow septum.33 Although LVOTO gradient is the most evident abnormality, the physiology of obstructive disease includes elevated left ventricular (LV) end-diastolic pressure, mitral regurgitation and a potential for abrupt changes in LVOTO magnitude (for example with postural change or sudden exertion); these abnormalities, as well as the increased LV afterload, may all contribute to symptoms.
A characteristic feature of LVOTO is that the severity of the obstruction is dynamic and subject to prevailing haemodynamic conditions. LVOTO is exacerbated by conditions causing reduced preload (e.g. Valsalva, squat to stand), reduced afterload (e.g. vasodilators) or positive inotropes.3 Notably, although LVOTO is most often seen in HCM, it may also occur in other conditions or physiological states and is not pathognomonic of HCM.34,35 In a smaller subgroup of HCM patients, obstruction can develop at the mid-left ventricular cavity. Mid-cavity obstruction is caused by the systolic apposition of hypertrophied mid-ventricular segments and/or papillary muscles creating a characteristic hour-glass appearance with a distinct apical cavity.36,37 This form of obstruction is often associated with apical aneurysm formation.36,37 Mid-cavity obstruction can exist in isolation (see Figure 2) or in conjunction with LVOTO. Even though ASA has been used in the treatment of mid-cavity obstruction,38,39 this is not routine practice and further discussion is beyond the scope of this review.
Assessment of Left Ventricular Outflow Tract Obstruction
All patients with HCM should undergo a detailed transthoracic echocardiogram to examine: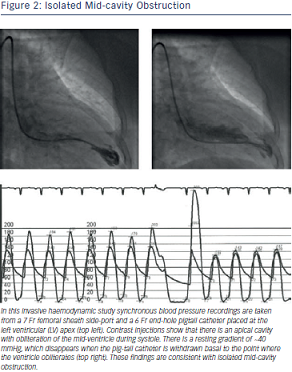
If the transthoracic echocardiogram fails to provide the necessary diagnostic information, a transoesophageal echocardiogram, CMR or an invasive haemodynamic study may be required.
An instantaneous Doppler LVOT gradient of ≥30 mmHg is considered significant, and such patients are classified as having the obstructive form of the disease. However, LVOTO is considered to be haemodynamically significant only when the LVOT gradient is ≥50 mmHg.3 There are few data to support these thresholds,3 which are largely empirically defined and reflect an understanding that when LVOTO is mild, therapeutic reduction of LVOTO is less likely to improve symptoms, and alternative causes of severe symptoms should be sought.
Since LVOTO is dynamic, obstruction may not be apparent in the supine patient under resting conditions. Exercise, the Valsalva manoeuvre, postural changes or an isoprenaline infusion help demonstrate latent LVOTO.6,40–42 These additional tests are mandatory in the evaluation of patients with symptoms suggestive of obstruction, which is not demonstrable at rest.